Mogroside aerosol and preparation method thereof
A mogroside and inhalation powder spray technology, applied in the field of medicine, can solve the problems of unsatisfactory treatment effect and the like, and achieve the effects of low clinical use price, rapid absorption and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Investigation on the preparation process of mogroside superfine powder
[0039]In order to better deposit the drug in the lungs, the drug particles in the inhaled powder should be mainly distributed in the range of 1 μm to 5 μm. The fourth part of the "2015 Chinese Pharmacopoeia" stipulates that the particle size of raw materials in inhalation preparations should be controlled below 10 μm, and most of them should be below 5 μm. After inhalation, particles with too small particle size diffuse in the form of Brownian motion in bronchioles and alveoli and other parts with slow airflow, making it difficult to settle, and are easy to be exhaled with the expiratory airflow.
[0040] In this experiment, the superfine powder of mogroside was prepared by wet ball milling, and the preparation process of mixed drug powder was optimized by star-point design-response surface method. E40) 8g, placed in a 100ml stainless steel ball mill jar, the grinding medium is stainless...
Embodiment 2
[0043] Example 2 Selection of Lactose in the Preparation Technology of Mogroside Inhalation Powder
[0044] Mogroside MFC-E40 (Gifuss (China) Luo Han Guo Co., Ltd.), lactose for inhalation 120 (Germany MEGLE Group), 230 (Megel Group, Germany), 400 (German MEGLE Group), 3 # Hypromellose Empty Capsules (Suzhou Capsule Co., Ltd.)
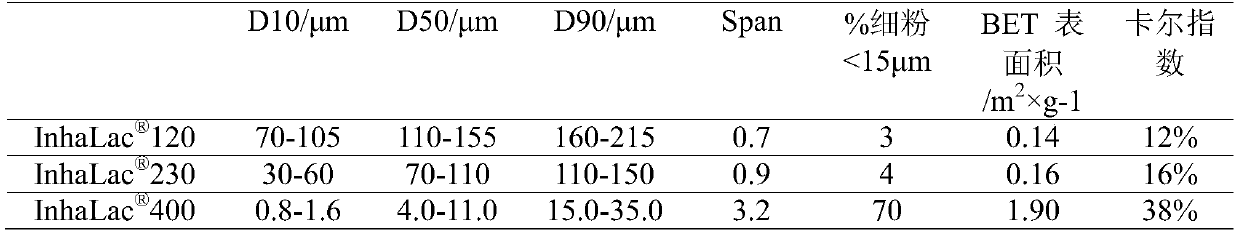
[0045] The particle size distribution and fluidity of lactose for inhalation used in this research are shown in the table below, 120、 The particle size distribution range of 230 is larger than 400, the content of fine powder with a particle size of less than 15 μm is also less, so the fluidity is better, and it has a stronger effect on improving the fluidity of mogroside inhalation powder. 120、 230.
[0046] Table 1 Particle size distribution and fluidity of lactose for inhalation
[0047]
Embodiment 3
[0048] Example 3 Fluidity Evaluation in the Preparation Process of Mogroside Inhalation Powder
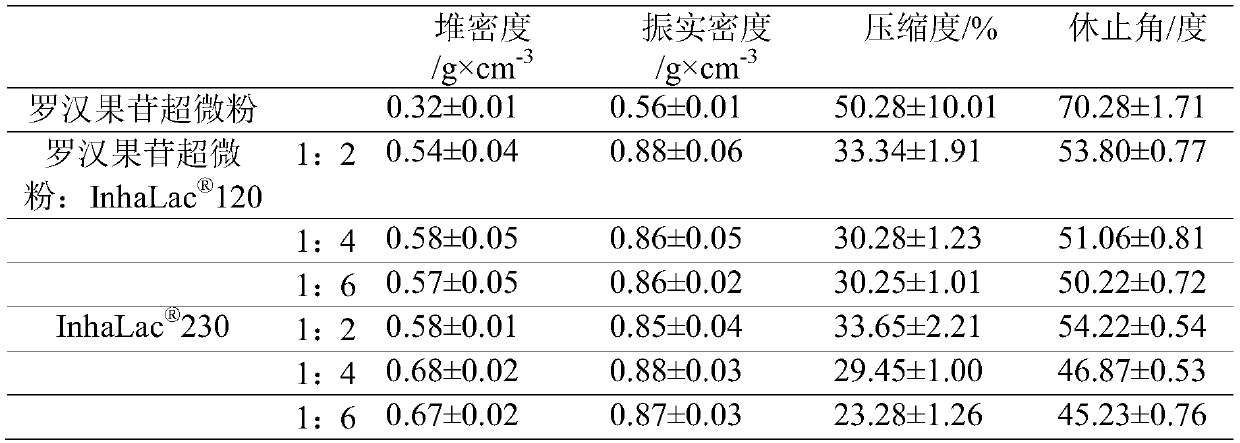
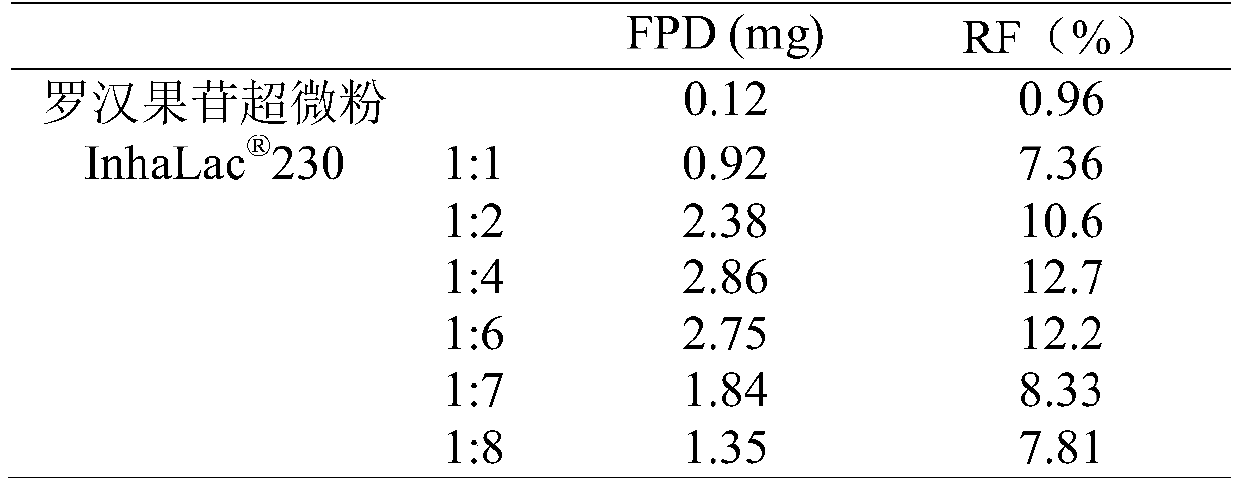
[0049] Mogroside superfine powder and carrier lactose 120、 230 were mixed according to 1:2, 1:4, 1:6 respectively, passed through 80-mesh sieve 10 times, and mixed well to obtain. A total of 6 different prescriptions of mogroside inhalation powder and aerosol pharmaceutical powder were prepared, and the fluidity measurement results of each prescription are shown in Table 2.
[0050] Table 2 Different prescription fluidity measurement results (n=3)
[0051]
[0052] It can be seen from Table 2 that adding lactose can significantly improve the fluidity of mogroside superfine powder. Mogroside superfine powder is very easy to aggregate and agglomerate, and has a high angle of repose (70.28 degrees), and its fluidity is expressed by the degree of compression, which can reach 50.28%, indicating that the fluidity is extremely poor. joining In the prescription of 120, as the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Maximum particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com