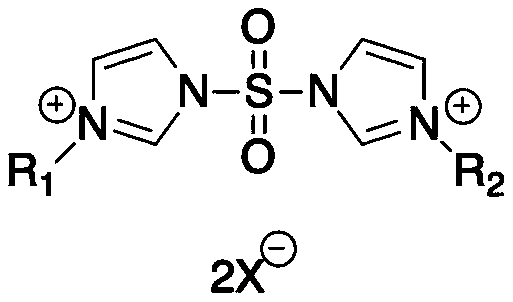
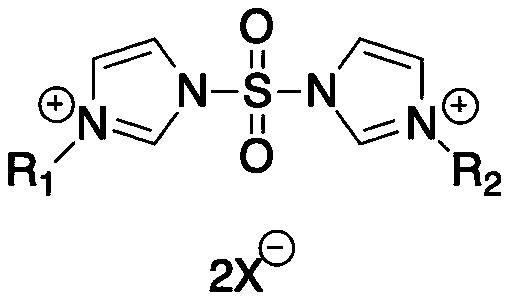
Preparation method of sulfonyldiimidazole-based ionic liquid
A technology of sulfuryl diimidazolium and sulfuryl diimidazole, which is applied in the field of preparation of sulfuryl diimidazolium-based ionic liquids, can solve problems affecting battery cycle performance, structure collapse, and transition metal ion dissolution, etc., and achieve novel structure and improved High voltage resistance and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 400g of dichloromethane into a 1000mL two-necked flask, then put 200g of imidazole into it, stir to make it fully dissolve, and place the reaction bottle in a low-temperature reactor at 0°C, then gradually add 86.2g of it dropwise to the reaction bottle Sulfonyl chloride (obtained according to the method of non-patent literature: J. Power Sources, 2016, 329, 586-593), gas is released during the reaction, and the hydrogen chloride gas generated is absorbed by aqueous sodium hydroxide solution. After the dropwise addition was completed, the reaction temperature was adjusted to room temperature and the reaction was stopped after stirring for 15 h. After filtering and washing twice with dichloromethane, the dichloromethane was spin-dried to obtain a light yellow solid, which was crystallized with boiling isopropanol to obtain 113.9 g of white crystals, namely sulfuryl diimidazole, with a yield of 90%.
[0026] Continue to dissolve the sulfuryl diimidazole obtained above...
Embodiment 2
[0029] Add 400g of dichloromethane into a 1000mL two-necked flask, then put 200g of imidazole into it, stir to make it fully dissolve, and place the reaction bottle in a low-temperature reactor at 0°C, then gradually add 86.2g of it dropwise to the reaction bottle Sulfonyl chloride (obtained according to the method of non-patent literature: J. Power Sources, 2016, 329, 586-593), gas is released during the reaction, and the hydrogen chloride gas generated is absorbed by aqueous sodium hydroxide solution. After the tape addition was completed, the reaction temperature was adjusted to room temperature and the reaction was stopped after stirring for 15 h. After filtering and washing twice with dichloromethane, the dichloromethane was spin-dried to obtain a light yellow solid, which was crystallized with boiling isopropanol to obtain 113.9 g of white crystals, namely sulfuryl diimidazole, with a yield of 90%.
[0030] Continue to dissolve the sulfuryl diimidazole obtained above int...
Embodiment 3
[0033] Add 400g of dichloromethane into a 1000mL two-necked flask, then put 200g of imidazole into it, stir to make it fully dissolve, and place the reaction bottle in a low-temperature reactor at 0°C, then gradually add 86.2g of it dropwise to the reaction bottle Sulfonyl chloride (obtained according to the method of non-patent literature: J. Power Sources, 2016, 329, 586-593), gas is released during the reaction, and the hydrogen chloride gas generated is absorbed by aqueous sodium hydroxide solution. After the dropwise addition was completed, the reaction temperature was adjusted to room temperature and the reaction was stopped after stirring for 15 h. After filtering and washing twice with dichloromethane, the dichloromethane was spin-dried to obtain a light yellow solid, which was crystallized with boiling isopropanol to obtain 113.9 g of white crystals, namely sulfuryl diimidazole, with a yield of 90%.
[0034] Continue to dissolve the sulfuryl diimidazole obtained above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com