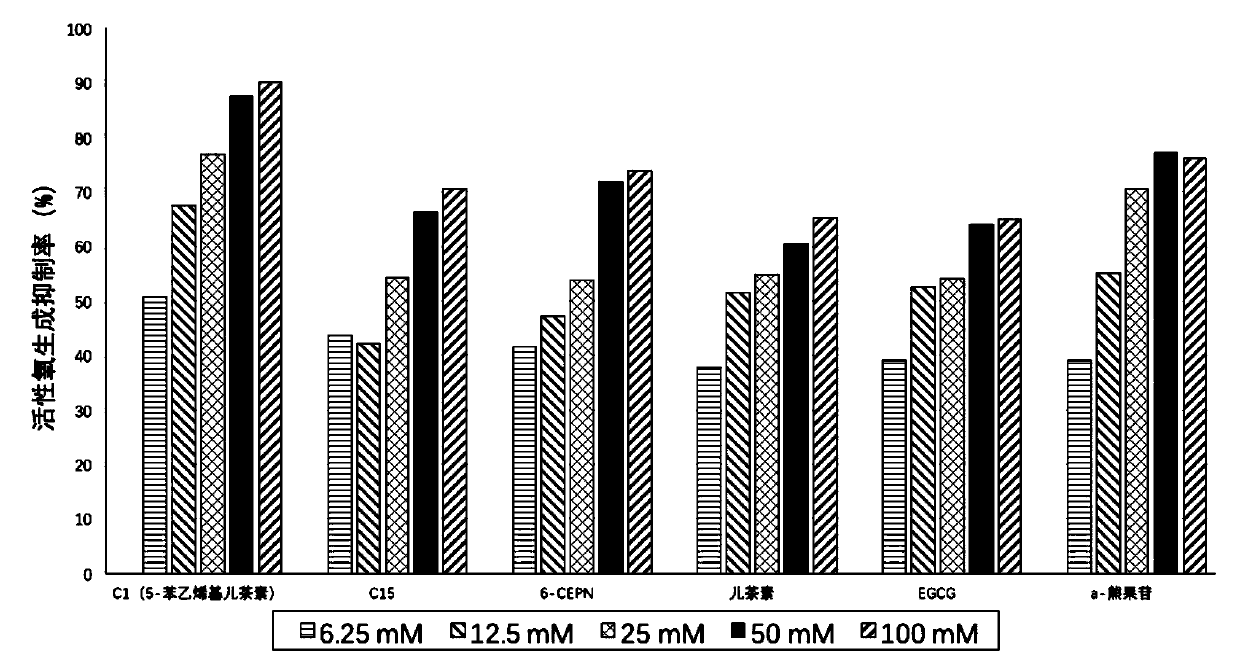
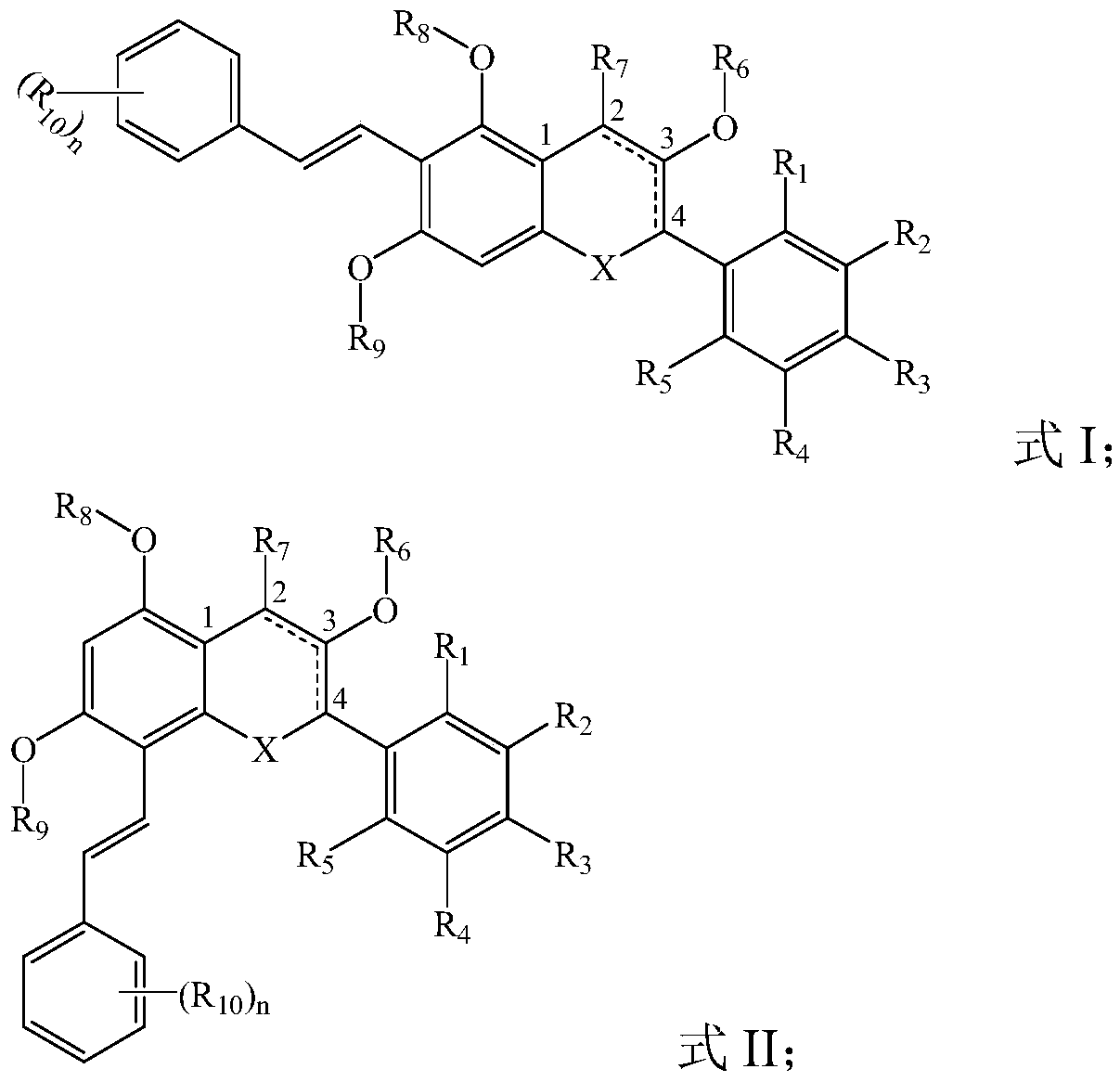
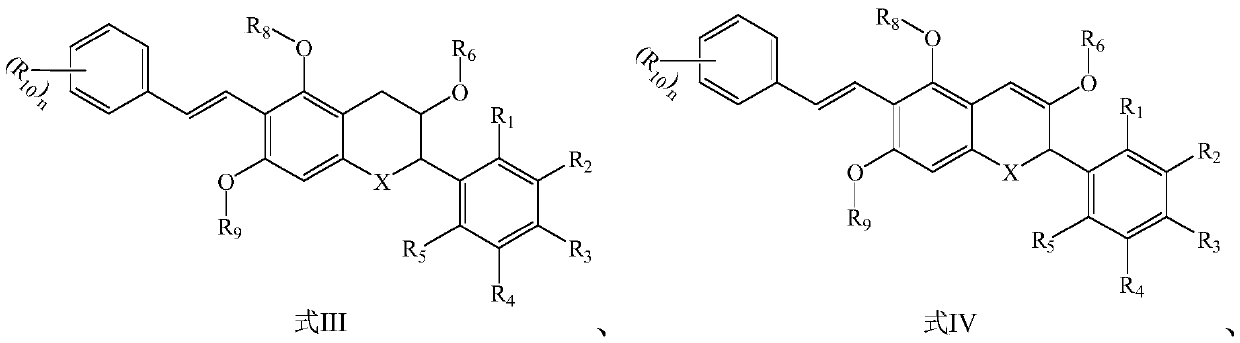
Flavone derivative, preparation method and application thereof, and whitening product containing flavone derivative
A technology of derivatives and flavonoids, applied in the field of daily chemical products, can solve the problems of low whitening effect of natural flavonoids and difficulty in meeting market demands.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of compound C1 (5-styryl catechin) and compound D1 (7-styryl catechin), the specific synthesis steps are as follows:
[0072] In a 100mL round bottom flask, the raw material catechin (580mg, 2mmol, 1eq) or epicatechin (580mg, 2mmol, 1eq), and phenylacetaldehyde (600μL, 2.5eq) were added to diethylene glycol (40mL ), add 5.3% hydrochloric acid (14mL) dropwise, heat and react at 120°C for 2 hours, after the reaction is complete, remove the solvent in vacuo, add ethyl acetate (50mL) to dissolve, wash with water (20mL×3), and separate the organic phase , the organic phase was concentrated to dryness in vacuo, and separated by column chromatography (selecting 200-300 mesh silica gel, the eluent is a mixed solution of dichloromethane and methanol, volume ratio 20:1), to obtain the colorless oily target product, According to the results of column chromatography, C1 (5-styryl catechin) was firstly obtained with a yield of 75%, and then D1 (7-styryl catechin) was...
Embodiment 2
[0078] For the preparation of compound C2, the specific synthetic steps differ from those in Example 1 in that catechin was replaced by 3',4'-dimethoxycatechin, and the yield was 52%.
[0079] Structure Characterization:
[0080] 1 H NMR (400MHz, CDCl 3 ), δ=7.72(m,2H),7.44(m,2H),7.30-7.21(m,2H),6.97-6.96(m,2H),6.87-6.77(m,2H),5.98(s,1H ),6.52(d,1H),5.82(s,1H),5.02(s,1H),4.89-4.88(m,2H),3.83(s,3H),3.75(s,3H),2.86-2.81( m,2H).
[0081] HRMS(ESI-ion trap)m / z:[M+H]+calculated value C 25 h 24 o 6 420.1573, found 420.1572.
Embodiment 3
[0083] The preparation of compound C3, the specific synthesis steps are as follows:
[0084] (1) In a 100mL round-bottomed flask, the raw materials afucatechin (540mg, 2mmol, 1eq) and thionyl chloride (600μL, 2.5eq) were added to 1,4-dioxane (20mL), 1 mL of dimethylformamide was added dropwise and reacted at room temperature for 12 hours. After the reaction was completed, the solvent was removed in vacuo to obtain 4'-chloroafucatechin;
[0085] (2) In a 100mL round bottom flask, add raw materials 4'-chloroafucatechin (600mg, 1eq) and phenylacetaldehyde (600μL, 2.5eq) into diethylene glycol (40mL), dropwise 5.3% hydrochloric acid (14mL), heat reaction at 140°C for 2 hours, after the reaction, remove the solvent in vacuo, add ethyl acetate (50mL) to dissolve, wash with water (20mL×3), separate the organic phase, and concentrate the organic phase in vacuo To dryness, use column chromatography to separate (choose 200-300 mesh silica gel, the eluent is a mixture of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com