Preparation method of voglibose
A technology of voglibose and compounds, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production, expensive palladium, and heavy metal pollution, and achieve the goals of reducing safety risks and product quality risks, excellent product quality, and reducing raw material costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Voglibose (Compound II)
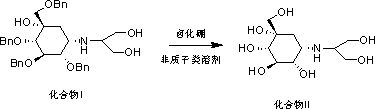
[0028] Put 100g of tetrabenzyl voglibose into a 3L three-necked bottle, add 1000ml of dichloromethane to dissolve, control the temperature at 15~30℃, add 180g of boron tribromide dropwise, and react for 60~90min after the completion of the reaction , control the temperature at 15~30℃, slowly add 96.7g of triethylamine dropwise, after dropping, cool down to 0~5℃, filter with suction, add 400ml 90% isopropanol to the filter cake and heat to 80~86℃ to dissolve. , naturally cooled to room temperature, suction filtered, and dried under reduced pressure at 40° C. to obtain 33 g of white solid. Yield: 77.6%, purity 99.939%, residue on ignition 0.08%.
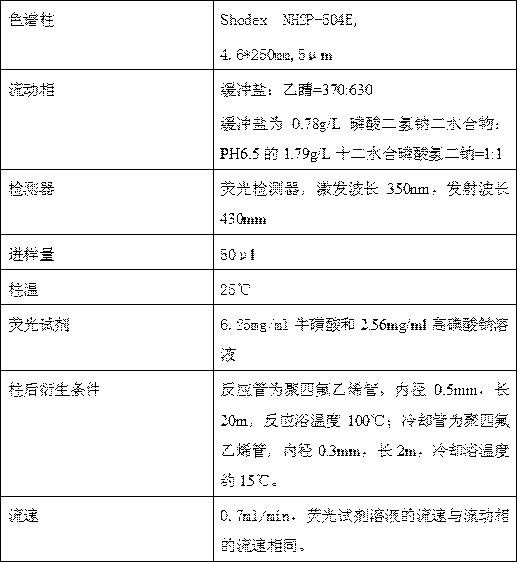
[0029] HPLC Detection Conditions
[0030]
[0031] result:
[0032]
[0033] Detector A Ex: 350nm, Em: 430
[0034] peak number keep time area high area% Number of theoretical plates (USP) Tailing factor Resolution (USP) 1 9.573 134401 5024...
Embodiment 2
[0035] Example 2 Preparation of Voglibose (Compound II)
[0036] Take 100g of tetrabenzyl voglibose and put it into a 3L three-necked bottle, add 1200ml of tetrahydrofuran, start stirring to dissolve, control the temperature to 15~30°C, add 104g of boron trifluoride diethyl ether dropwise, dropwise, after the reaction is complete, Control the temperature at 15~30°C, add 105g of diisopropylamine dropwise, cool down to 0~5°C, filter with suction, add 450ml of 90% ethanol to the filter cake, heat to 78~85°C, keep stirring for 30~40min, cool down to 0 ~5°C, filter with suction, and dry the filter cake under reduced pressure at 40°C to obtain 35.3 g of white solid with a purity of 99.930% and a yield of 82.86%.
[0037] HPLC Detection Conditions
[0038]
[0039] result:
[0040]
[0041] Detector A Ex: 350nm, Em: 430
[0042] peak number keep time area high area% Number of theoretical plates (USP) Tailing factor Resolution (USP) 1 9.576 166339 ...
Embodiment 3
[0043] Example 3 Preparation of Voglibose (Compound II)
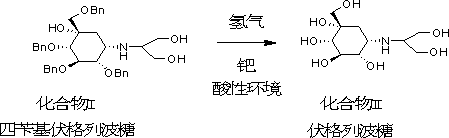
[0044] Take 100g of tetrabenzyl voglibose and put it into a 2L hydrogenation kettle, add 600ml of tetrahydrofuran, 600ml of methanol, 10g of 5% palladium carbon, add hydrogen until the pressure reaches 6-8kg, start stirring, and control the temperature to 15~30℃ for 24 Hours, after the reaction is complete, control the temperature at 15~30°C, add 105g of diisopropylamine dropwise, cool down to 0~5°C, filter with suction, add 450ml of 90% ethanol to the filter cake, heat to 78~85°C, keep stirring for 30 After ~40min, cool down to 0~5°C, filter with suction, and dry the filter cake under reduced pressure at 40°C to obtain 34.0g of white solid with a purity of 99.4% and a yield of 79.8%.
[0045] HPLC Detection Conditions
[0046]
[0047] result:
[0048]
[0049] Detector A Ex: 350nm, Em: 430
[0050] peak number keep time area area% Number of theoretical plates (USP) Tailing factor Resolutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com