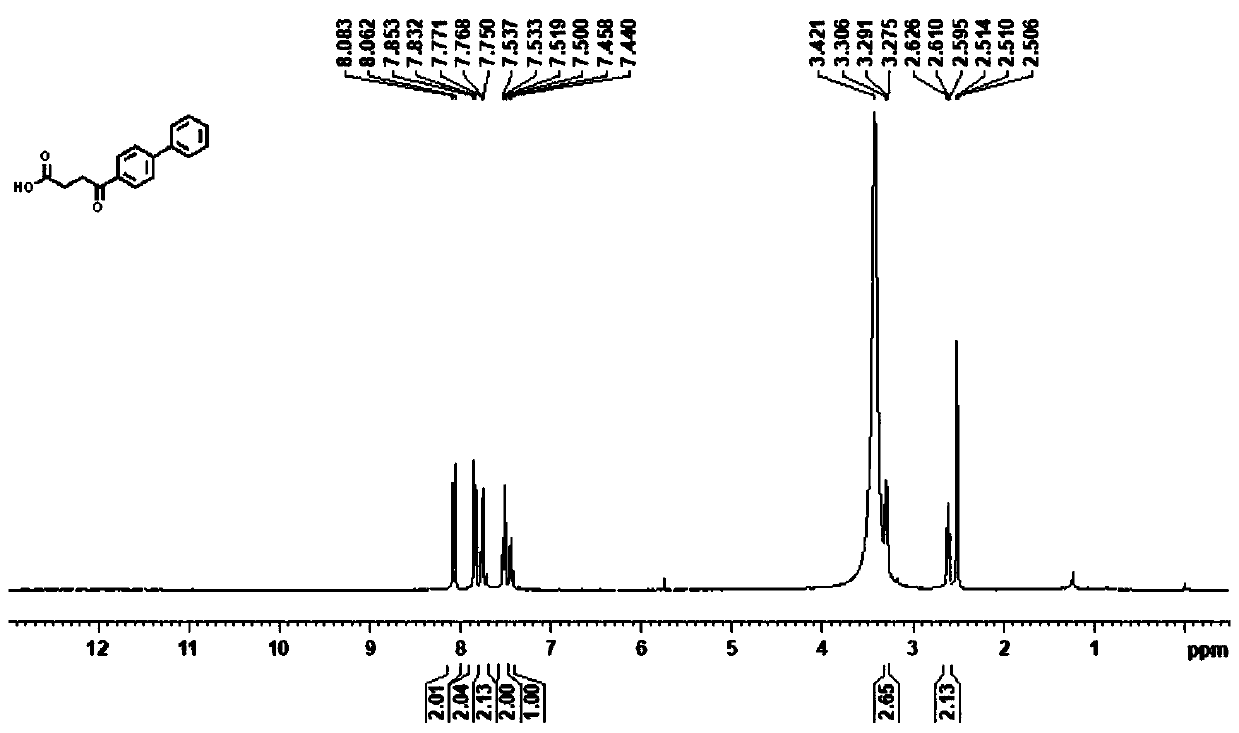
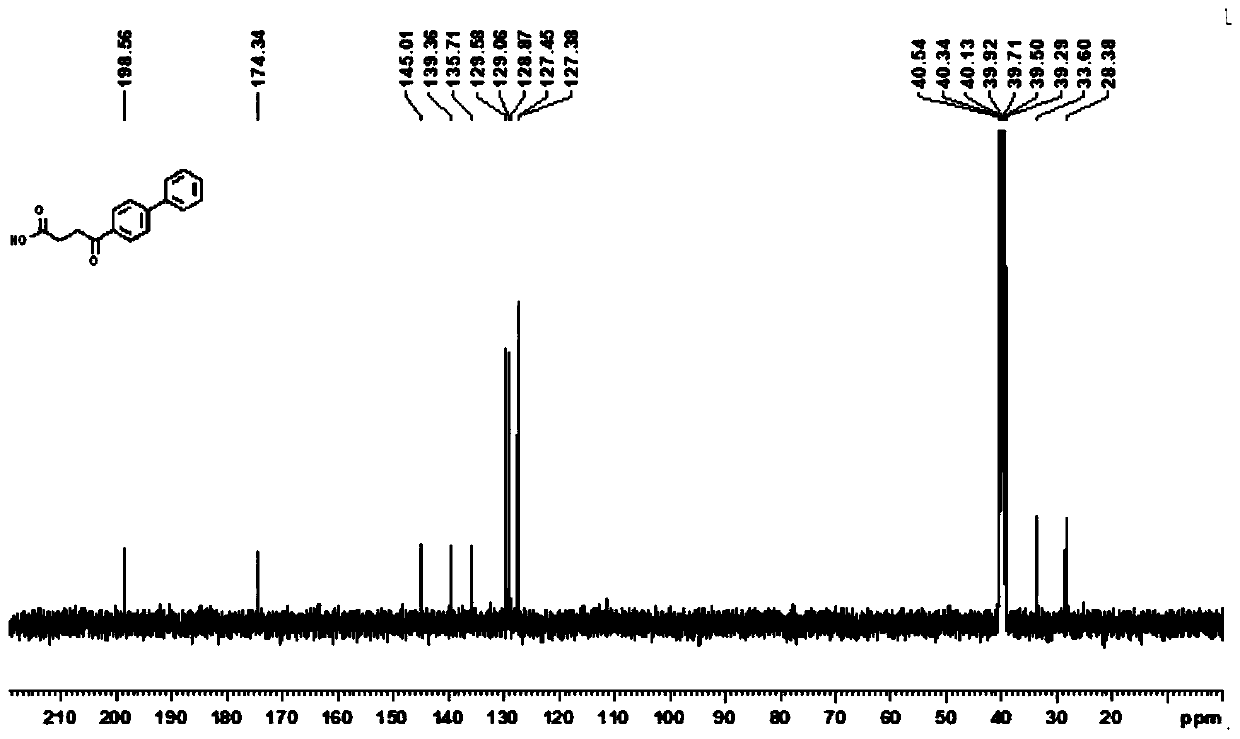
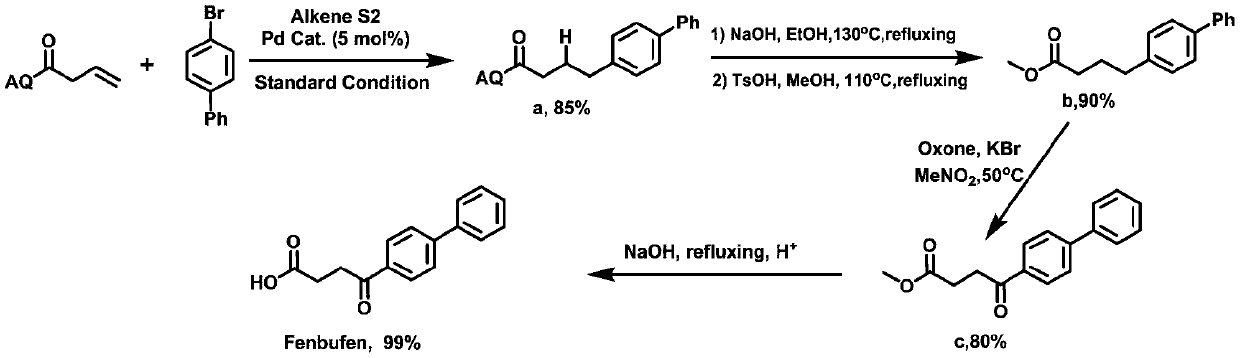
Synthesis method of Fenbufen
A synthesis method and compound technology, applied in chemical instruments and methods, pharmaceutical formulations, drug combinations, etc., can solve problems such as low product yield, complicated post-treatment purification process, and mild reaction conditions, and achieve reaction and post-treatment purification Simple process, high reaction regioselectivity and yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Under an inert atmosphere, mix 0.01mmol allylpalladium(II) chloride dimer, 0.2mmol 2-(dicyclohexylphosphono)-1-phenyl-1H-pyrrole, 1mmol N-(octa Aminoquinoline) but-3-enamide, 5mmol lithium acetate, 3mmol 4-bromobiphenyl, 3mmol cyanoacetic acid, and 100mmol water were mixed, and 0.1mmol of the mixed material and 1mL of anhydrous 2,3-butanediol were added to the reaction vessel Mix well in the medium, place the reaction vessel in an oil bath at 125°C and stir vigorously for 12 hours, and purify the reaction product through a silica gel column (flushing the chromatography silica gel column with petroleum ether at a ratio of ethyl acetate 1:20), to obtain Group compounds;
[0024] (2) 1 mmol of the compound is added to 50 mmol of ethanol solvent containing 1.5 mmol of sodium hydroxide, and the mixture is heated to 130° C. for reflux for 12 hours, and the reaction product is distilled under reduced pressure (the pressure of the reduced pressure distillation is within 100...
Embodiment 2
[0028] (1) Under an inert atmosphere, mix 0.1mmol allylpalladium(II) chloride dimer, 0.02mmol 2-(dicyclohexylphosphono)-1-phenyl-1H-pyrrole, 1mmol N-(octa Aminoquinoline) but-3-enamide, 1mmol lithium acetate, 1)mmol 4-bromobiphenyl, 1mmol cyanoacetic acid and 1mmol water were mixed, and 0.1mmol mixed material and 1mL anhydrous 2,3-butanediol were added to Mix well in the reaction vessel, place the reaction vessel in an oil bath at 125°C and vigorously stir the reaction for 12 hours, and purify the reaction product through a silica gel column (flush the chromatographic silica gel column with petroleum ether at a ratio of ethyl acetate 1:20), to obtain Compounds with directing groups;
[0029] (2) 1mmol of the compound is added to 5mmol ethanol solvent containing 4mmol sodium hydroxide, and the mixture is heated to 140°C for 12 hours under reflux, and the reaction product is subjected to underpressure distillation (the pressure of underpressure distillation is within 100mbar, th...
Embodiment 3
[0033] (1) Under an inert atmosphere, mix 0.05mmol allylpalladium(II) chloride dimer, 0.1mmol 2-(dicyclohexylphosphono)-1-phenyl-1H-pyrrole, 1mmol N-(octa Aminoquinoline) but-3-enamide, 3mmol lithium acetate, 2mmol 4-bromobiphenyl, 2mmol cyanoacetic acid, and 50mmol water were mixed, and 0.1mmol of the mixed material and 1mL of anhydrous 2,3-butanediol were added to the reaction vessel Mix well in the medium, place the reaction vessel in an oil bath at 125°C and stir vigorously for 12 hours, and purify the reaction product through a silica gel column (flushing the chromatography silica gel column with petroleum ether at a ratio of ethyl acetate 1:20), to obtain Group compounds;
[0034] (2) 1 mmol of the compound is added to 30 mmol of ethanol solvent containing 2.5 mmol of sodium hydroxide, and the mixture is heated to 135° C. for reflux for 12 hours, and the reaction product is subjected to underpressure distillation (the pressure of underpressure distillation is within 100 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com