Synthetic method 6-amino-1-hexanol
A synthesis method and hexanol technology are applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of carbamate derivatives, etc., and can solve the problems of harsh reaction conditions, potential safety hazards, difficult to meet, etc., and achieve fewer reaction steps and cost. Low, short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 78 g of chlorosulfonyl isocyanate and 250 ml of toluene to the reaction flask, cool to 0°C, slowly add 18 g of methanol dropwise, and stir for 30 minutes; maintain this temperature, add 112 g of triethylamine dropwise, and continue stirring for 1 hour. Add 59 grams of 1,6-hexanediol, first stir the reaction at room temperature for 30 minutes, then raise the temperature to 50°C for 2 hours.
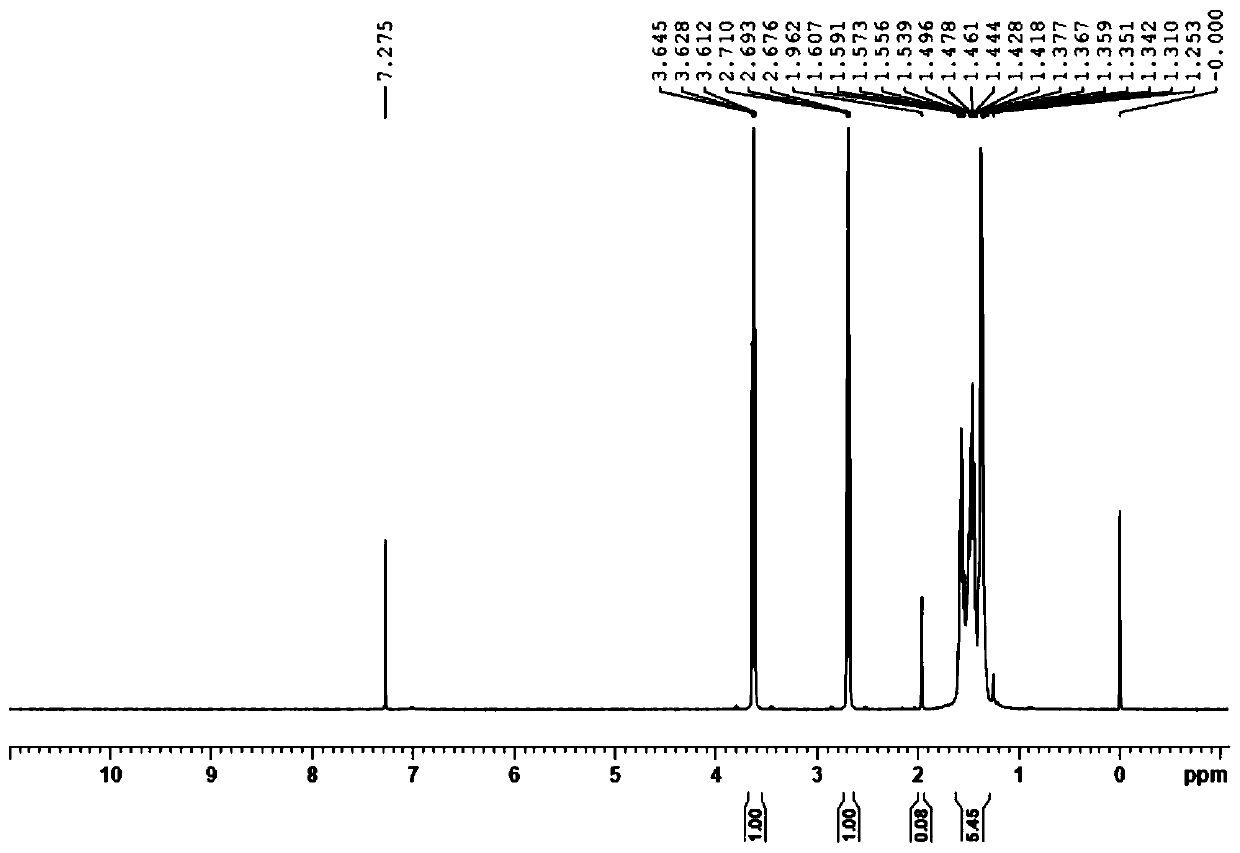
[0027] Suction filtration, add 100 ml of concentrated hydrochloric acid to the filtrate, heat and reflux for 3 hours, cool, separate the liquid, concentrate the water phase to dryness, use solid sodium hydroxide for alkalization, extract 3 times with tetrahydrofuran, combine the organic phases, concentrate, and the remaining The product was distilled under reduced pressure, and the 70-78°C / 1-2mmHg fraction was collected to obtain 35 grams of 6-amino-1-hexanol, with a yield of 60% and a purity of 98.2%; 1 H (NMR, CDCl3): 3.59 (t, 2H), 2.69 (t, 2H), 2.23 (brs, 3H), 1.59-1.52 (m, 2...
Embodiment 2
[0029] Add 78 grams of chlorosulfonyl isocyanate and 250 milliliters of toluene to the reaction flask, cool to 10°C, slowly add 41 grams of tert-butanol dropwise, and stir for 30 minutes; maintain this temperature, add 112 grams of triethylamine dropwise, and continue stirring for 1 hour . Add 59 grams of 1,6-hexanediol, first stir the reaction at room temperature for 30 minutes, then raise the temperature to 85°C for 2 hours.
[0030] Suction filtration, add 50 milliliters of trifluoroacetic acid to the filtrate, stir at room temperature for 12 hours, then add 100 milliliters of water to fully stir, separate liquids, concentrate the water phase to dryness, use solid sodium hydroxide for alkalization, and extract 3 times with tetrahydrofuran, The organic phases were combined and concentrated, and the residue was distilled under reduced pressure, and the 70-78°C / 1-2mmHg fraction was collected to obtain 38 g of 6-amino-1-hexanol with a yield of 65% and a purity of 98.8%.
Embodiment 3
[0032] Add 78 grams of chlorosulfonyl isocyanate and 250 milliliters of toluene to the reaction flask, cool to 15°C, slowly add 60 grams of benzyl alcohol dropwise, and stir for 30 minutes; maintain this temperature, add 112 grams of triethylamine dropwise, and continue stirring for 1 hour. Add 59 grams of 1,6-hexanediol, first stir the reaction at room temperature for 30 minutes, then raise the temperature to 85°C for 2 hours.
[0033] Suction filtration, add 100 ml of 40% hydrobromic acid to the filtrate, azeotropic dehydration reaction for 4 hours, then add 50 ml of water and stir thoroughly, separate liquids, concentrate the water phase to dryness, use solid sodium hydroxide for alkalization, and extract with tetrahydrofuran Three times, the organic phases were combined and concentrated, and the residue was distilled under reduced pressure, and the 70-78°C / 1-2mmHg fraction was collected to obtain 46 grams of 6-amino-1-hexanol with a yield of 79% and a purity of 98.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com