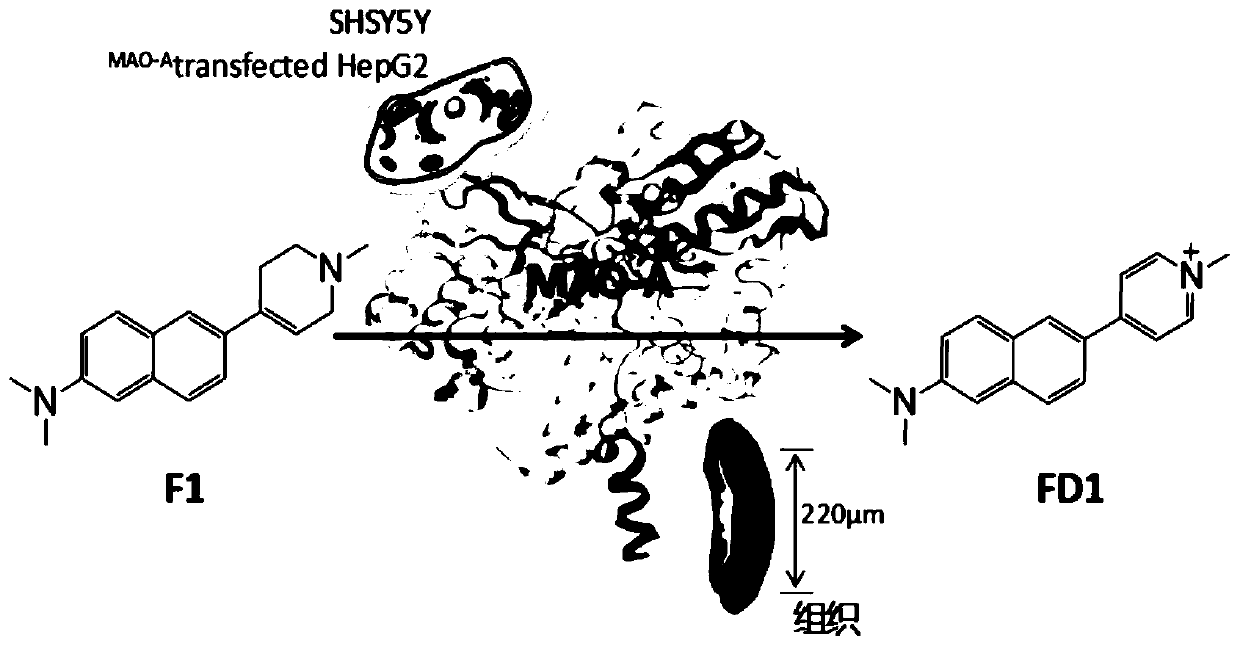
Specific two-photon fluorescent probe of monoamine oxidase A, and preparation method and application thereof
A monoamine oxidase and two-photon fluorescence technology, applied in the field of organic fluorescent probes, can solve problems such as inability to organize imaging, achieve good fluorescence properties, a simple and easy synthesis process, and reduce biological background fluorescence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The chemical synthesis method of embodiment 1 F1
[0036] The reaction formula is as follows:
[0037]
[0038] The first step: 6-bromo-N, N-dimethylnaphthalene-2-amine
[0039] A mixture of dimethylamine solution (2 mL, 30.16 mmol), 6-bromo-2-naphthol (1.0 g, 4, 48 mmol), Na2S2O5 (1.78 g, 9.37 mmol) and H2O (15 mL) in a microwave tube was Stir at 160°C for 7 hours. The product was washed with sodium chloride solution, the aqueous phase was extracted with ethyl acetate, then purified by flash column chromatography, and spin-dried to obtain 6-bromo-2-dimethylaminonaphthalene as a white solid, namely compound 1, with a yield of 0.71 g, 63%. 1 H NMR (500MHz, CDCl 3 )δ7.84 (s,1H),7.84(s,1H),7.62(d,J=9.1Hz,1H),7.58(dd,J=25.5,8.9Hz,2H), 7.53(d,J=8.7 Hz,1H),7.43(d,J=8.7Hz,1H),7.43(d,J=8.7Hz,1H),7.33 –7.09(m,2H),7.19(d,J=9.8Hz,1H), 6.93(s,1H),6.93(s,1H),3.06(s,6H), 3.06(s,7H). 13 C NMR (75MHz, CDCl 3 )δ=151.30, 136.07, 131.99, 130.47, 130.44, 119.69, 117.72, 108.84, ...
Embodiment 2
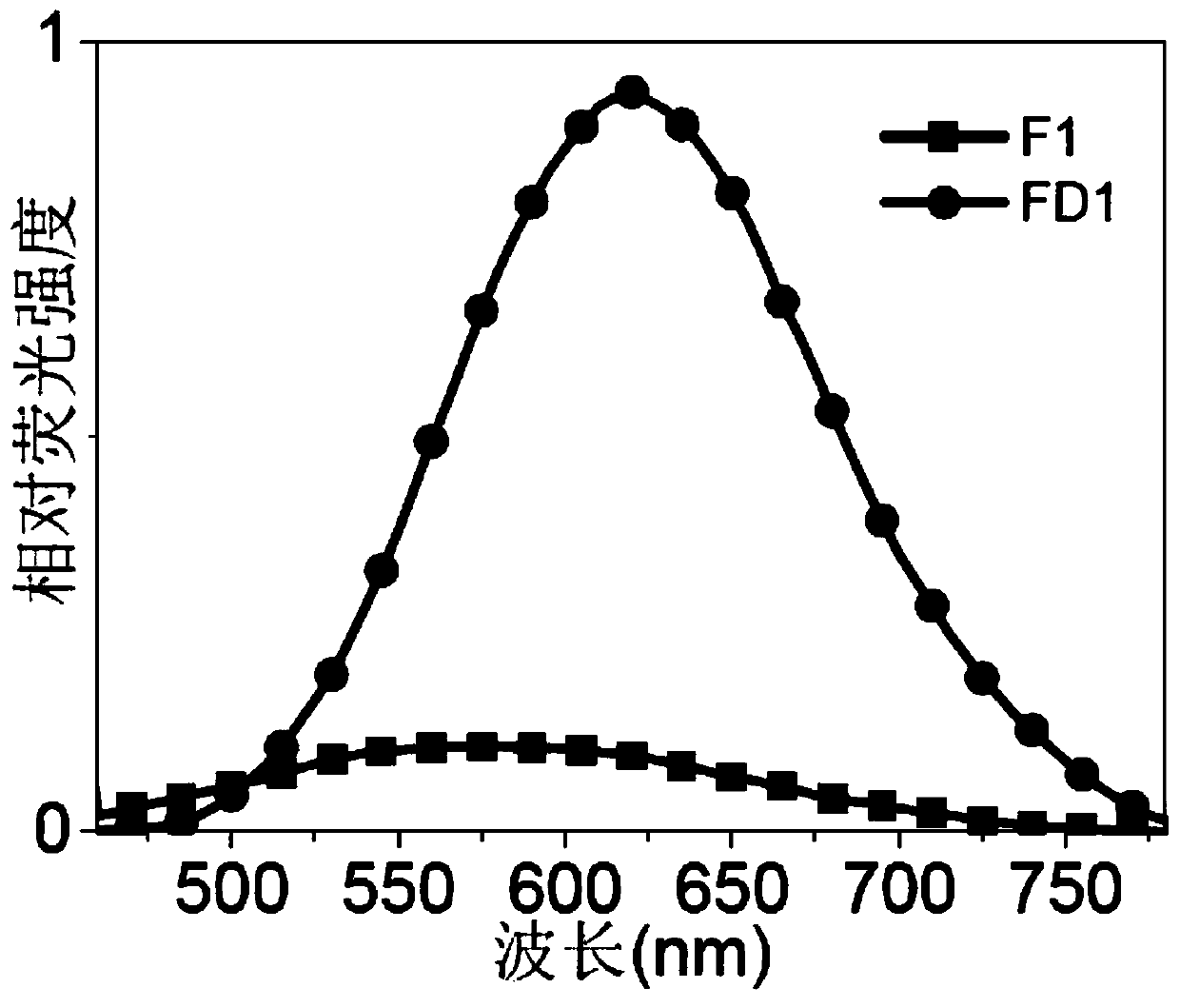
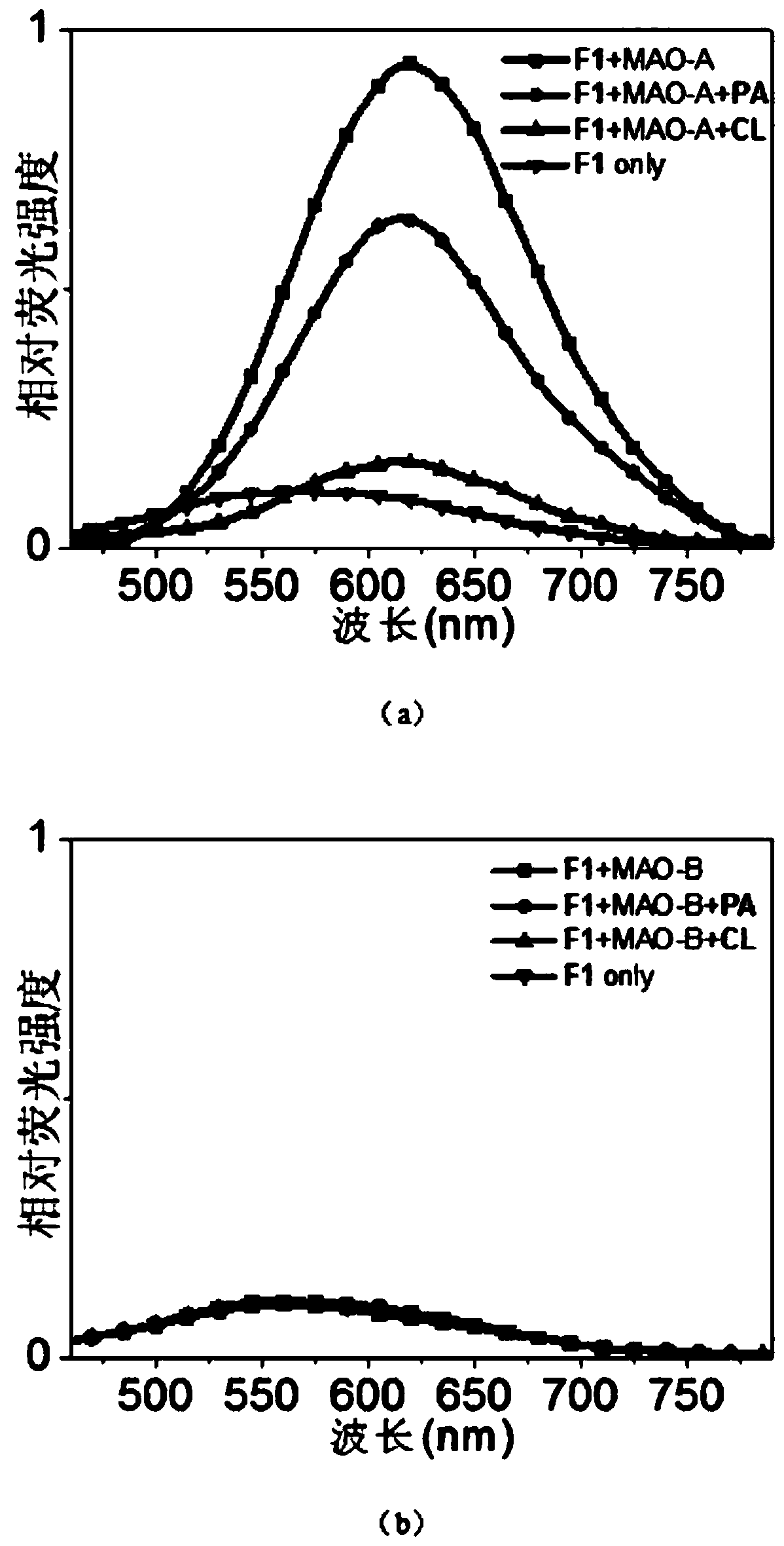
[0048] Embodiment 2 monoamine oxidase concentration curve
[0049] (1) Prepare 200 μL of monoamine oxidase reaction system in advance, including PBS buffer with pH=7.4, 0.4% Triton X-100, monoamine oxidase A / B (0-10 μg / mL), F1 (1 μM), at 37 ° C Pre-incubate with shaking for 1 hour.
[0050] (2) Fluorescence scanning detection (Ex=430nm) is carried out after reacting for 1 hour, and the fluorescence intensity increases with the concentration of monoamine oxidase A, see Figure 4 .
Embodiment 3
[0051] Activity detection of monoamine oxidase A in the cell lysate of embodiment 3
[0052] (1) SH-SY5Y / HepG-2 cells were inoculated in a 10 cm culture dish, and the medium was Dulbecco's modified Eagle medium (DMEM), containing 10% fetal bovine serum (FBS), 100.0 mg / L streptomycin and 100IU / mL penicillin. When the cell density is about 90%, pour out the culture medium in the culture dish, wash once with PBS buffer (pH=7.4), then add 1 mL of PBS buffer (pH=7.4) with 0.4% Triton X-100, The cells were broken with a cell scraper, and then the cell lysate was collected, centrifuged at 4° C. and 12000 rpm for 10 minutes, and the supernatant was taken to obtain a clarified cell lysate.
[0053] (2) Determination of total protein concentration in lysate
[0054] The total protein concentration of the lysate was determined using the BCA protein concentration assay kit.
[0055] (3) Prepare 200 μL of cell lysate reaction system, including PBS buffer with pH=7.4, 0.4% TritonX-100, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com