Preparation method of duck reovirus inactivated vaccine
A technology of reovirus and inactivated vaccine, which is applied in the field of preparation of duck reovirus inactivated vaccine, can solve the problem of uncontrollable epidemic situation, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
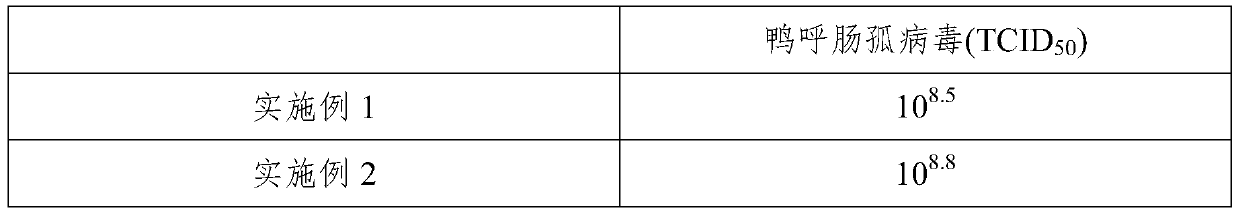
Embodiment 1
[0036] Embodiment 1, a kind of preparation method of duck reovirus inactivated vaccine
[0037] Preparation of S1 cell carrier: Digest and disperse LMH cells with trypsin, and use DMEM / F12 culture medium containing 5% newborn bovine serum and 1000 units / mL penicillin-streptomycin double antibody at 37°C, 5% CO 2 Cultured under the conditions of 2 days, then washed the cells twice with serum-free DMEM / F12 culture medium to obtain cell carriers; the LMH cells were cultured in spinner bottles or cultured in microcarrier reactors, and the amount of microcarriers used was 4 g / Lift;
[0038] Inoculation of S2 virus: inoculate the duck reovirus liquid into the cell carrier obtained in step S1 according to the final volume of 1:1000, place it at 37°C for 30 minutes, absorb the virus liquid, then add medium A, and incubate at 37°C , 5%CO 2 48h under the condition of culturing, 80% cytopathy occurs, and diseased cells are obtained;
[0039] Described culture medium A comprises DMEM / ...
Embodiment 2
[0047] Embodiment 2, a kind of preparation method of duck reovirus inactivated vaccine
[0048] Preparation of S1 cell carrier: Digest and disperse LMH cells with trypsin, and use DMEM / F12 culture medium containing 8% newborn bovine serum and 1500 units / mL penicillin-streptomycin double antibody at 37°C, 5% CO 2 Cultivate under the condition of 2 days, then wash cells 3 times with serum-free DMEM / F12 culture medium, obtain cell carrier; The described LMH cell is placed in the culture of microcarrier reactor, and microcarrier usage amount is 6 grams / liter;
[0049] Inoculation of S2 virus: Inoculate the duck reovirus liquid into the cell carrier obtained in step S1 according to the final volume of 1:2000, place it at 37°C for 40 minutes, then absorb the virus liquid, then add medium A, and incubate at 37°C , 5%CO 2 Under the conditions of 56 hours, 80% of the cells were damaged, and the diseased cells were obtained;
[0050] Described culture medium A comprises DMEM / F12 basal...
Embodiment 3
[0058] Embodiment 3, a kind of preparation method of duck reovirus inactivated vaccine
[0059] Preparation of S1 cell carrier: Digest and disperse LMH cells with trypsin, and use DMEM / F12 culture medium containing 10% newborn bovine serum and 2000 units / mL penicillin-streptomycin double antibody at 37°C, 5% CO 2 Cultivate under the condition of 3 days, then wash cells 3 times with serum-free DMEM / F12 culture medium, obtain cell carrier; Described LMH cell is placed in the culture of microcarrier reactor, and microcarrier usage amount is 8 grams / liter;
[0060] Inoculation of S2 virus: inoculate the duck reovirus liquid into the cell carrier obtained in step S1 according to the final volume of 1:4000, place it at 37°C for 60 minutes, then absorb the virus liquid, then add medium A, and incubate at 37°C , 5%CO 2 72h under the condition of culture, 80% cytopathy occurs, and diseased cells are obtained;
[0061] Described culture medium A comprises DMEM / F12 basal medium, also c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com