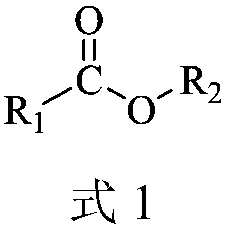
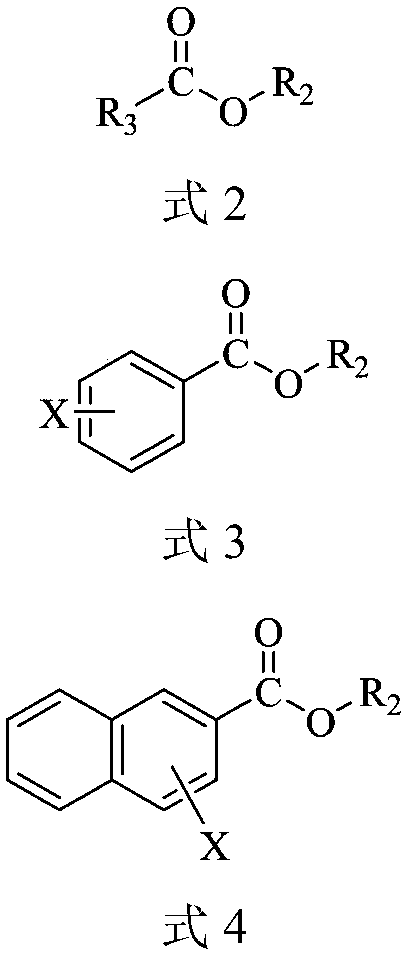
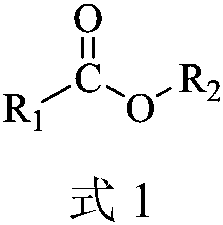
Carboxylic ester oximationmethod
A carboxylate, oximation reaction technology, applied in directions such as organic chemistry, can solve problems such as unfavorable industrial production of hydroxamic acid or its salt, high energy consumption, long time, etc., and achieves simplified industrial production process, short time and cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of benzoyl (different) sodium hydroxamate:
[0062] 21g sodium hydroxide is added in batches (6g, 5g, 4g, 3g, 3g) in the reactor that 19.2g hydroxylamine hydrochloride and 34g methyl benzoate are housed, fully mix, and keep feed temperature not exceeding 40 ℃, wait After all the sodium hydroxide was added, the temperature was raised to 50° C., and the reaction was carried out for 4 hours. After the reaction, the sodium benzoic acid (hydro)hydroxamate product was obtained. Based on the color reaction of ferric ions, the yield of sodium benzoic acid (hydro)hydroxamate was 98.6%.
Embodiment 2
[0064] Synthesis of Benzene (iso)hydroxamic acid:
[0065] 21g sodium hydroxide is added in batches (6g, 5g, 4g, 3g, 3g) in the reactor that 19.2g hydroxylamine hydrochloride and 34g methyl benzoate are housed, fully mix, and keep feed temperature not exceeding 40 ℃, wait After all the sodium hydroxide was added, the temperature was raised to 50° C., and the reaction was carried out for 4 hours. With sufficient mixing, the temperature of the reaction mixture was lowered below 10°C, and then 7mL of concentrated sulfuric acid was slowly added to the reactor while keeping the feed temperature below 10°C to obtain the benzyl (hydro)hydroxamic acid product based on ferric iron Ion chromogenic detection showed that the yield of benzoic acid (hydro)xamic acid was 98.3%.
Embodiment 3
[0067] Synthesis of Sodium Salicyl (iso)hydroxamic acid:
[0068] Compared with Example 1, the difference is only that, with 38g methyl salicylate instead of 34g methyl benzoate, the hydroximation reaction is carried out at 55° C. to prepare sodium salicylic (iso)hydroxamic acid by the similar technique of embodiment 1 , based on the detection of ferric ions, the yield of sodium salicyl (iso)hydroxamic acid was 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com