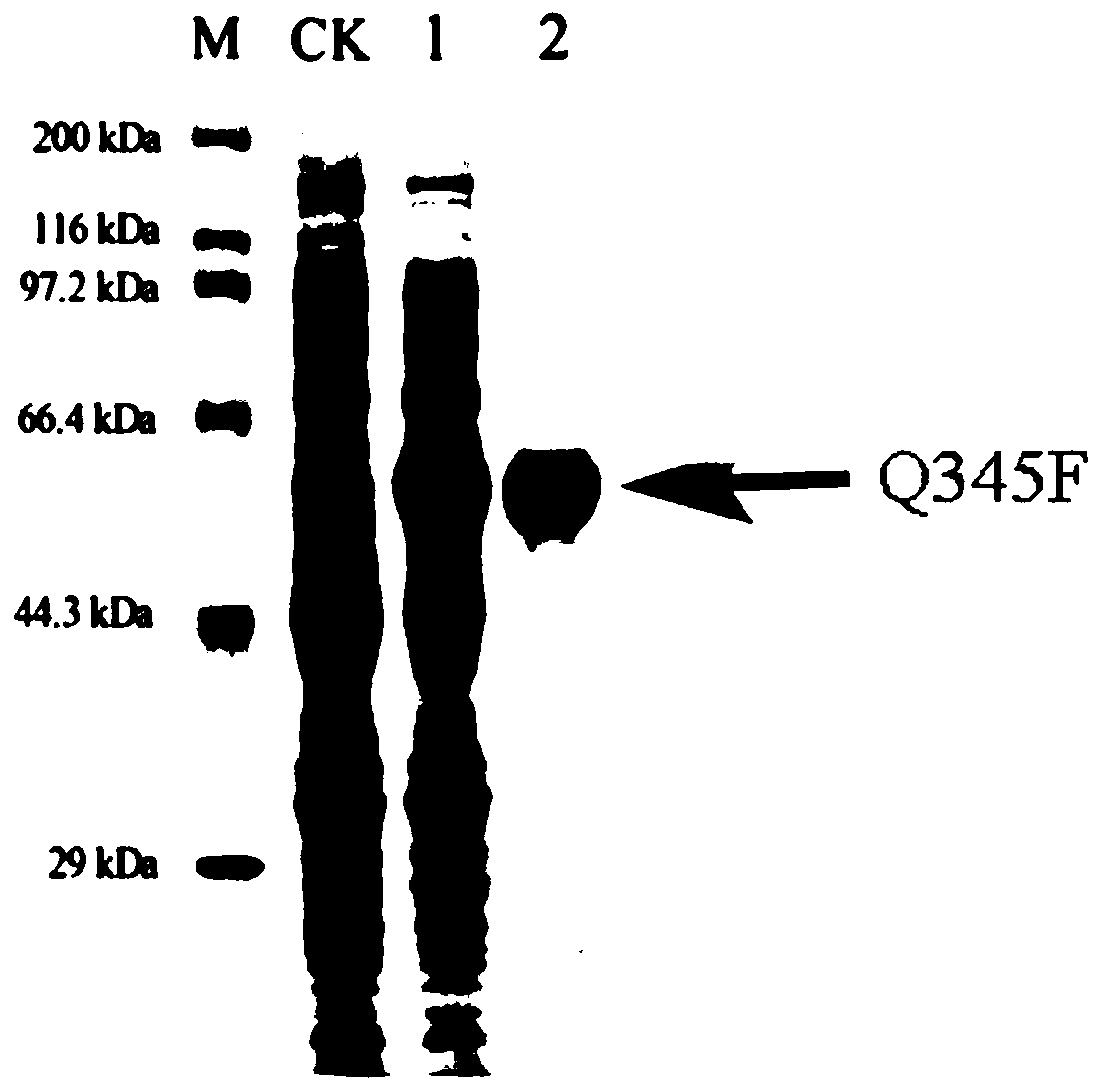
Sucrose phosphorylase mutant Q345F with glycosylation to quercetin
A technology of sucrose phosphorylase and mutants, which is applied in the field of enzyme molecular transformation, can solve the problems of poor quercetin effect, achieve high water solubility, and increase the effect of transglycosidic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Cloning of sucrose phosphorylase gene Bspase
[0023] A metagenomic library was constructed from soil samples collected from Wuming Sugar Factory in Guangxi, and a positive subclone pUC-SP with sucrose phosphorylase activity was screened from the metagenomic library. Sent to Huada Gene Company for DNA sequencing. The sequenced sequence was compared with Blastp and it was found that one of the ORFs had the highest homology with the sucrose phosphorylase gene from Bifidobacterium animalissubsp.lactis AD011 (Bifidobacterium lactis AD011). Concordance = 1521 / 1521 (100%), this ORF was named Bspase.
[0024]Design upstream primer Bspase-1 (comprising an EcoR I restriction site and six histidine tags) and downstream primer Bspase-2 (comprising a Hind III restriction site), using the subclone pUC-SP as a template, The sucrose phosphorylase gene Bspase was amplified by polymerase chain reaction PCR, and the sucrose phosphorylase Bspase was digested with restriction endonucl...
Embodiment 2
[0037] Transglycosidic activity and product analysis of quercetin by mutant enzyme Q345F:
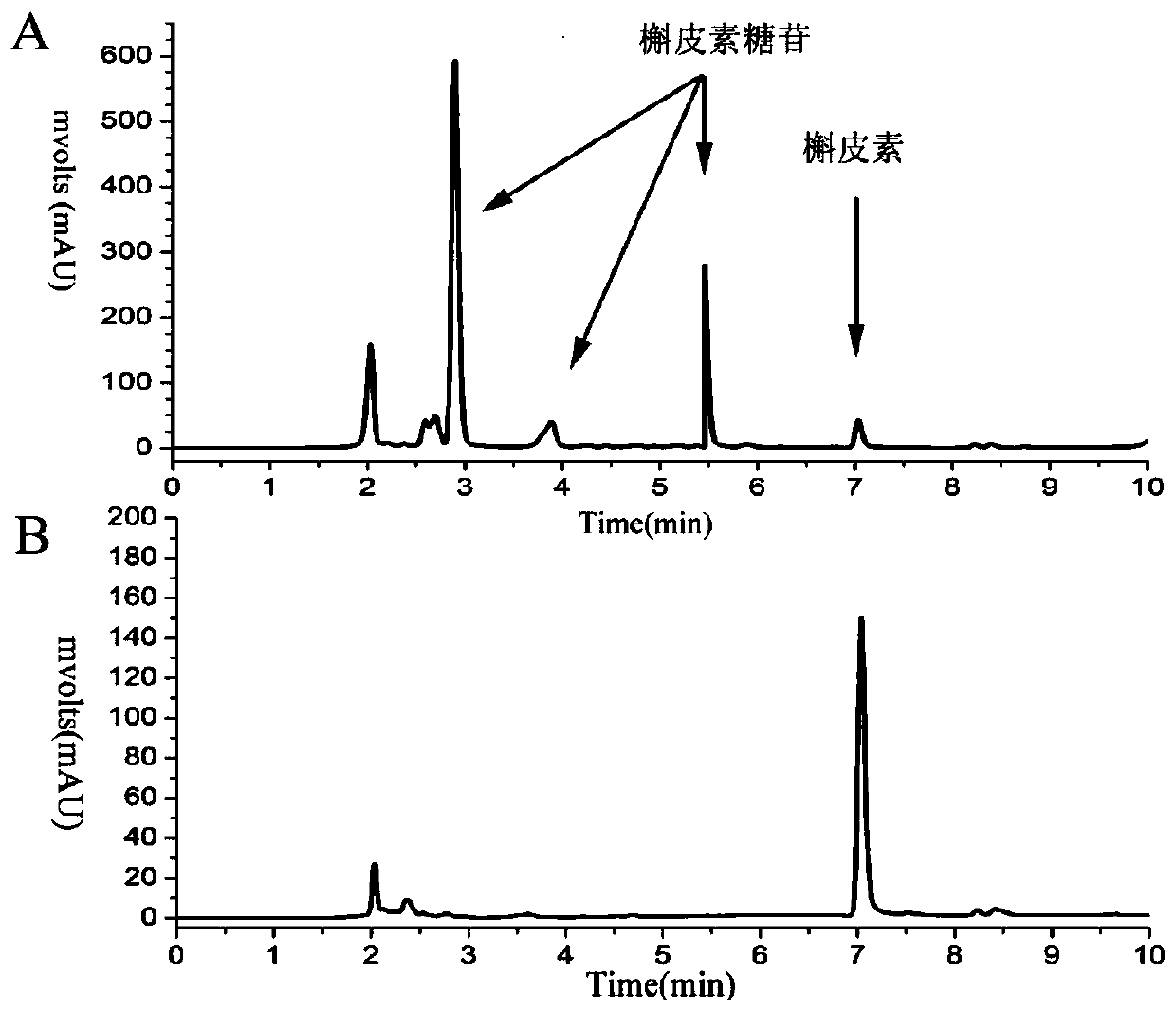
[0038] Under the condition of pH 7.0, 750mM sucrose was used as the glycosyl donor, 0.5% quercetin was used as the glycosyl acceptor, and 10% dimethyl sulfoxide (DMSO) was used as the water-insoluble quercetin and As the common solvent of the reaction system, the purified sucrose phosphorylase mutant Q345F obtained in Example 1 was added, and reacted at 37° C. for 24 hours. The reaction was terminated by boiling, cooled to room temperature and centrifuged at 12,000 rpm for 30 min to obtain the supernatant, and the product was analyzed by HPLC. The unmodified wild enzyme Bspase was used as a control to detect whether the mutant had the ability to glycosylate quercetin.
[0039] HPLC detection conditions of quercetin transglycoside products: Instrument: Agilent 1260 series liquid chromatograph, UV detector, mobile phase: A: water; B: methanol; C: acetonitrile, gradient elution: A:B:C= 6...
Embodiment 3
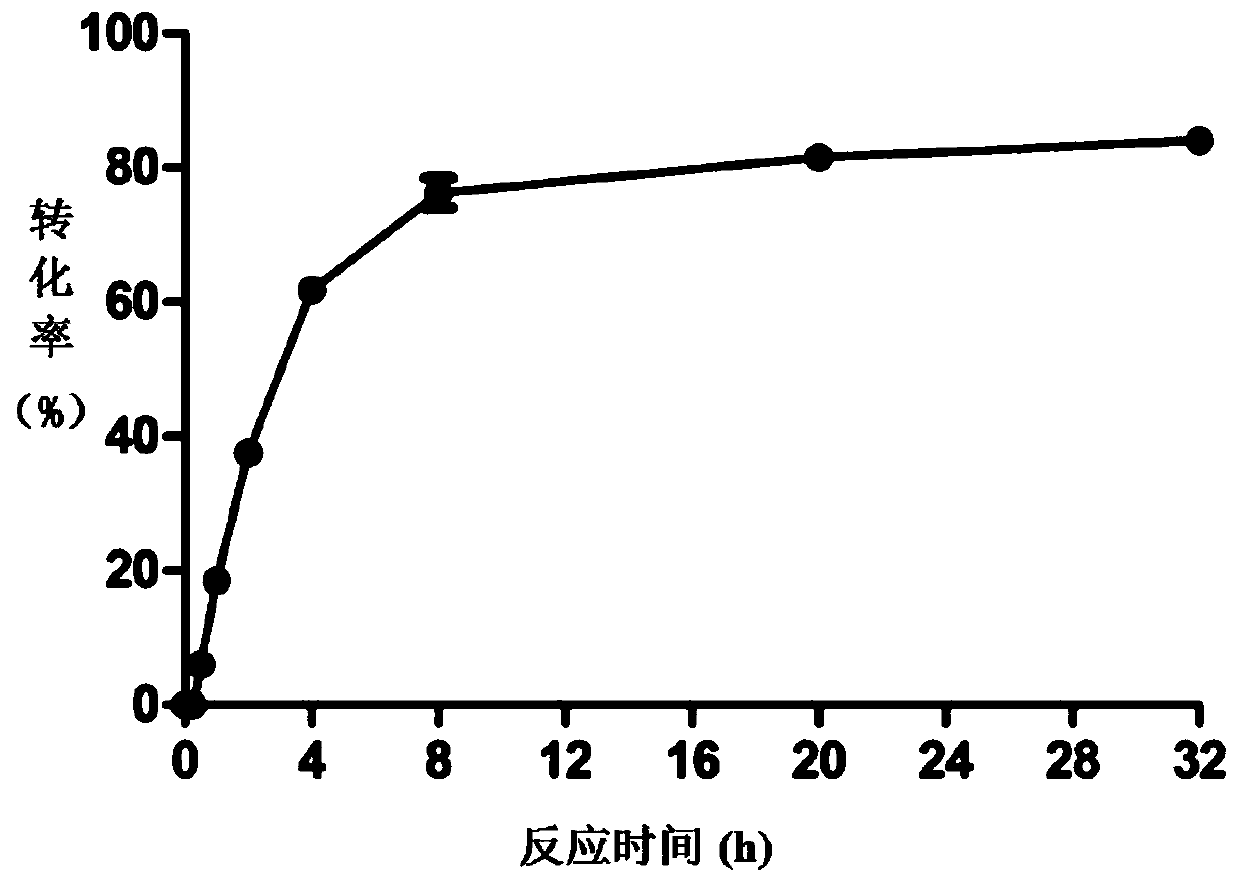
[0042] The reaction and detection method are the same as in Example 1, and the amount of purified sucrose phosphorylase mutant Q345F obtained in Example 1 is 200 μg / mL, and the reaction time interval sampling (0min, 15min, 30min, 1h, 2h, 4h, 8h, 20h, 32h), the reaction was terminated by boiling, and the reaction solution was diluted with 50% acetonitrile, filtered through a 0.2 μm organic filter membrane, and analyzed by HPLC. The effect of reaction time on the conversion rate is as image 3 shown.
[0043] From image 3 It can be seen that the maximum conversion efficiency is 85.3%, and the conversion efficiency of the mutant enzyme to quercetin is close to the maximum at about 8 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com