Method for preparing novel spiro compound through amino substituting
A reaction mixture and flange technology, applied in the field of pharmaceutical intermediates, can solve problems such as difficult to achieve high-speed stirring, short reaction time, damage to the whole bottle, etc., achieve good heating/cooling effect, stable stirring, and avoid loss effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
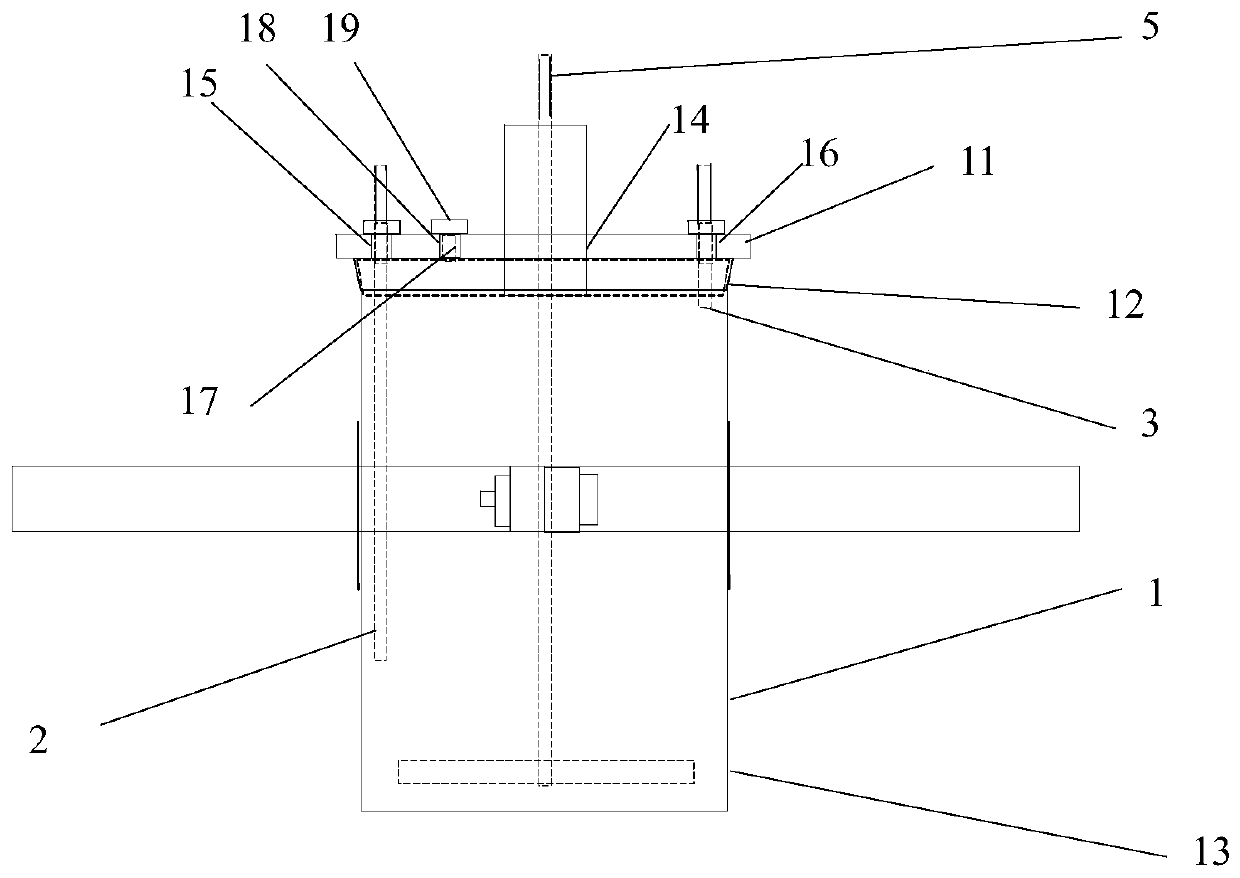
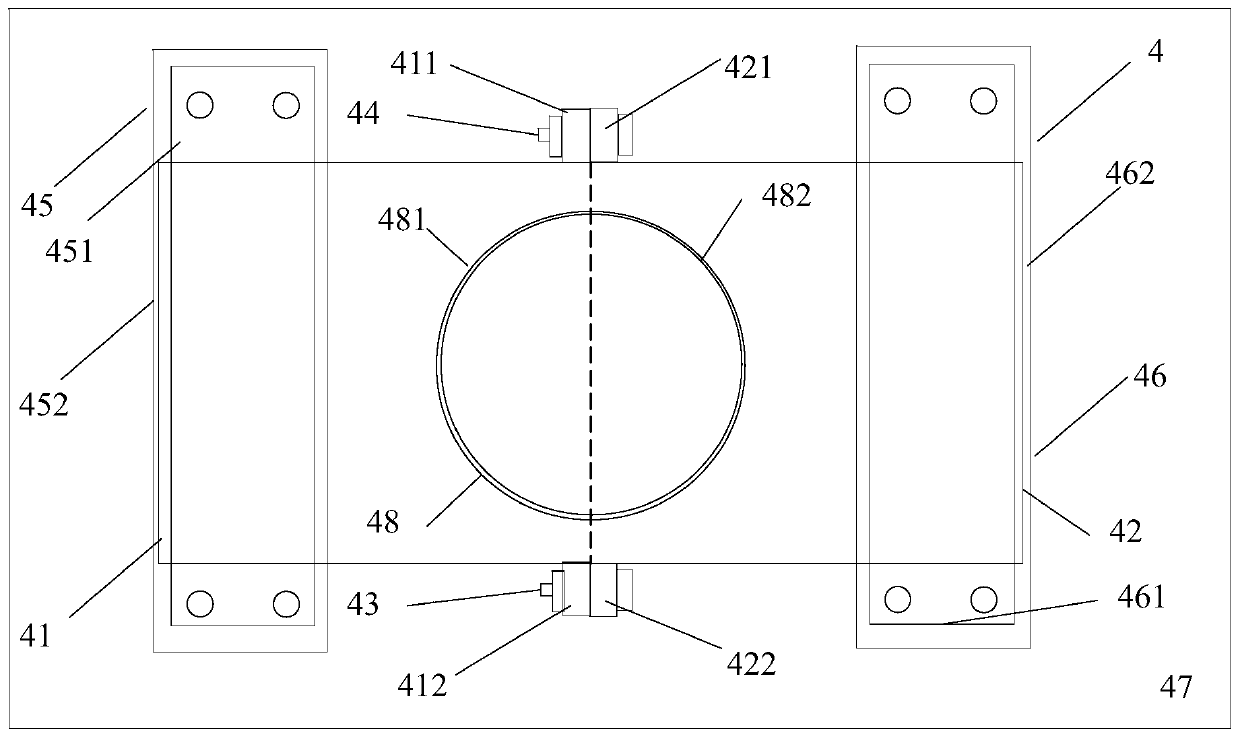
[0034] A reaction kettle, which has a body 1 made of glass, the body is equipped with a kettle cover 11, a lower flange 12, and a cylinder body 13. It is characterized in that: the reaction kettle also includes an air inlet combination 2, an air outlet combination 3, and a fixing device 4. Stirring section 5. The reaction kettle has improved the overall problems of the current four-neck bottle system as a whole: it is not stable enough, the bottom contact area is small, the heating and cooling are slow, the stirring speed cannot be increased, the nitrogen protection effect is not good, and the feeding must be completely opened, and it is very difficult to remove the reaction mixture. Troublesome and difficult to solve the problem.
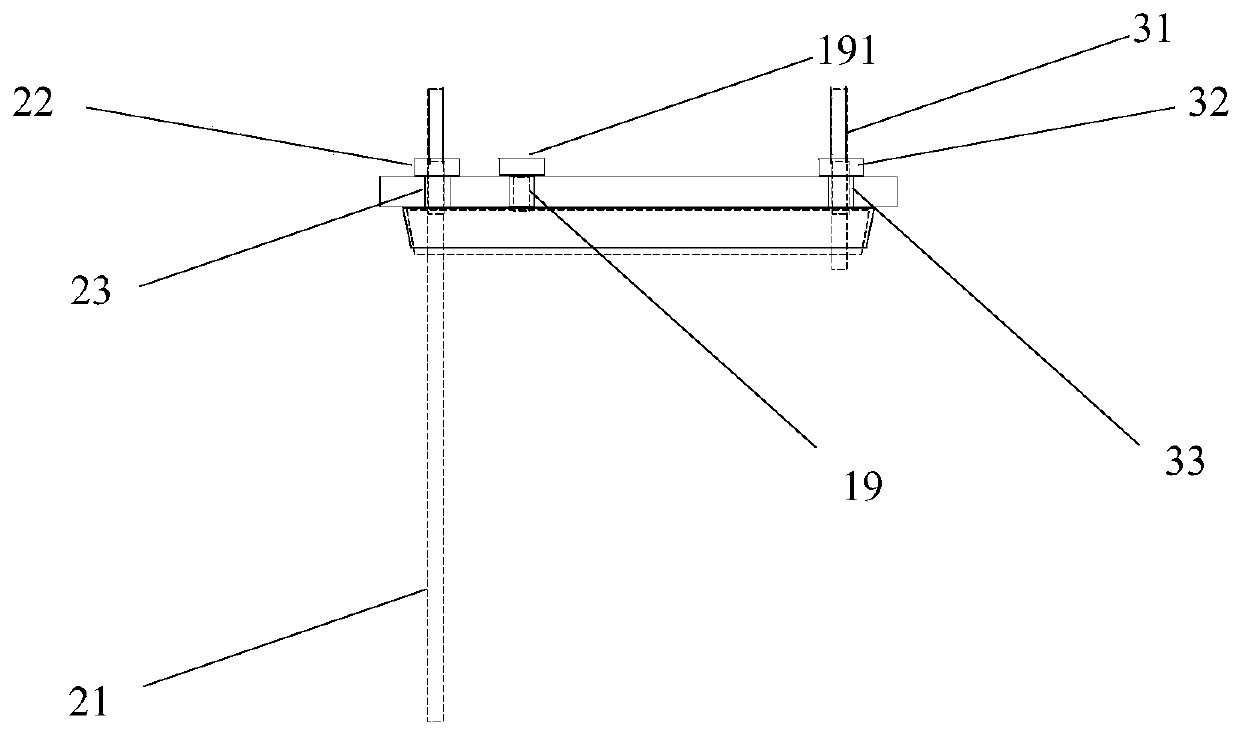
[0035] The center of kettle cover 11 has middle hole 14, and middle hole 14 is the circular through hole that the center is positioned at kettle cover 11 center places, and its inner surface is frosted shape, is used for bonding with the peripheral...
Embodiment 2
[0045] The preparation method of ((4-(aminomethyl)bicyclo[2.2.2]dec-1-yl)methyl)benzyl carbonate utilizes the reactor as described above to carry out, and the preparation steps are as follows.
[0046] 1) Under the protection of nitrogen, take a reaction kettle as mentioned above, clean and dry, fix the body (1) on the fixture (4), open the lid (11), and put 5.1g of 4-((( (Benzyloxy)carbonyl)amino)methyl)bicyclo[2.2.2]decane-1-carboxylic acid was dissolved in 20V of THF, the 4-(((((benzyloxy)carbonyl)amino)methyl) Bicyclo[2.2.2] decane-1-carboxylic acid hereinafter referred to as reactant A, add 3eq of reactant A NEt3, close the kettle lid (11), apply 150 rev / min rotating speed stirring to reaction mixture, with 180ml / Feed nitrogen into the min, open the feed plug (19), slowly drop the isobutyl chloroformate of 1.5eq of reactant A from the feed hole (18), after the dropwise addition, cover the feed plug (19), promote stirring Speed to 750 revs / min, after 1.2h, stop stirrin...
Embodiment 3
[0055] The preparation method of ((4-(aminomethyl)bicyclo[2.2.2]dec-1-yl)methyl)benzyl carbonate utilizes the reactor as described above to carry out, and the preparation steps are as follows.
[0056] 1) Under the protection of nitrogen, take a reaction kettle as mentioned above, clean and dry, fix the body (1) on the fixture (4), open the lid (11), and put 5.1g of 4-((( (Benzyloxy)carbonyl)amino)methyl)bicyclo[2.2.2]decane-1-carboxylic acid was dissolved in 20V of THF, the 4-(((((benzyloxy)carbonyl)amino)methyl) Bicyclo[2.2.2] decane-1-carboxylic acid is called reactant A below, adds the NEt of 3eq of reactant A3, closes still cover (11), the rotating speed stirring of 120-130 rev / min is applied to reaction mixture, with 140-160ml / min feeds nitrogen, opens feed plug (19), slowly drips the isobutyl chloroformate of 1.5eq of reactant A from feed hole (18), after dropwise, covers feed plug (19 ), promote the stirring speed to 680-700 rev / min, after 1h, stop stirring, take a ve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com