A kind of catalyst for the oxidation synthesis of benzaldehyde of benzyl alcohol and its preparation method and application
A technology for oxidative synthesis and benzyl alcohol, which is applied in catalyst activation/preparation, organic compound preparation, carbon-based compound preparation, etc. It can solve the problems of high cost and difficulty in large-scale use, and achieves reduced preparation costs and mild and simple preparation conditions , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of manganese dioxide / hydrothermal carbon composite microspheres
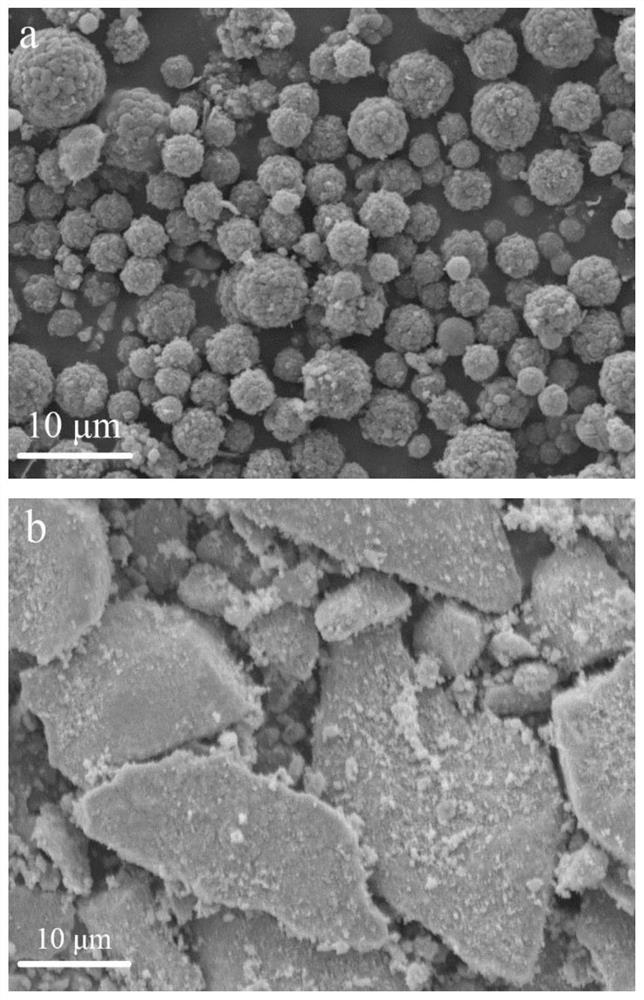
[0033]With 5.4g glucose (0.03mol), the manganese nitrate (0.06mol) of 1.0737g, the acrylic acid (0.003mol) of 0.216g, fully dissolve in 60ml deionized water, the above-mentioned mixed solution is placed in the hydrothermal reaction kettle, at 200 ℃ reaction 10h. After cooling, the mixture was washed with deionized water and absolute ethanol until the filtrate was neutral. Grind into powder after drying, take 1g of powder, put it in a beaker containing 100ml of deionized water, add 2g of potassium permanganate, put it on a magnetic stirrer and stir at a speed of 300rpm for 6h, and wash the mixture with deionized water until Neutral, manganese dioxide / hydrothermal carbon composite microspheres are obtained after drying.
[0034] (2) Evaluation of catalytic activity
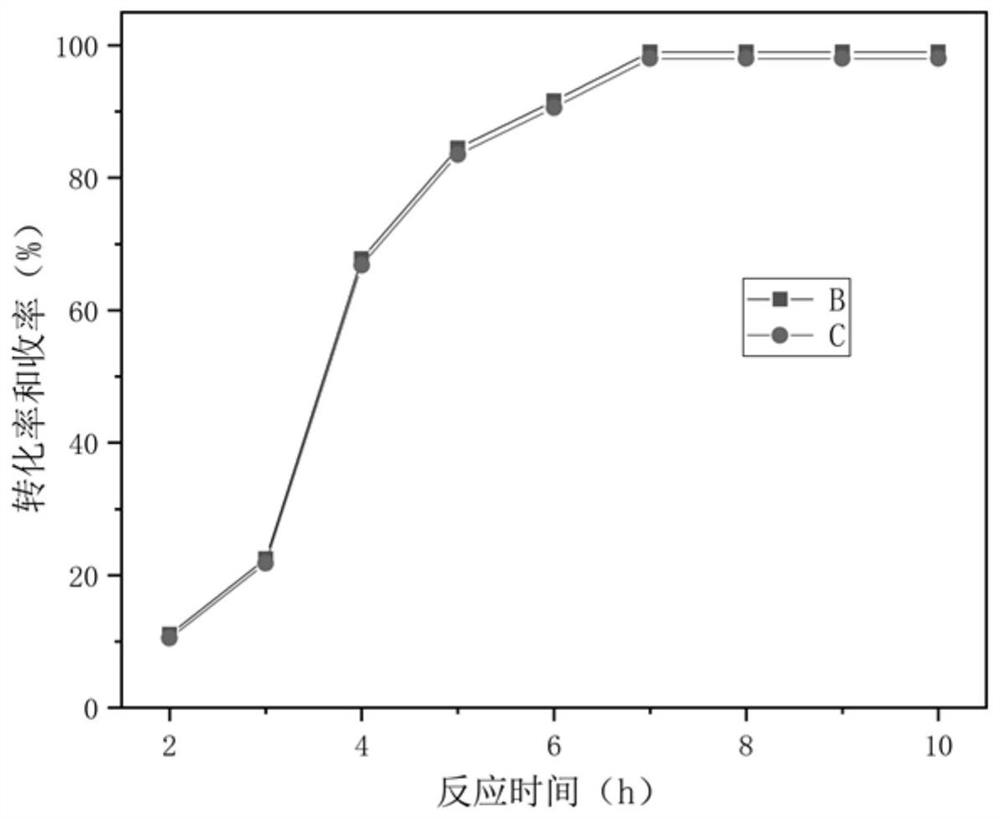
[0035] Get 0.108g benzyl alcohol (0.001mol), 9.2g toluene (0.1mol), the hydrogen peroxide solution (0.002mol) of 0.227g (30...
Embodiment 2
[0040] (1) Preparation of manganese dioxide / hydrothermal carbon composite microspheres
[0041] 10.3g sucrose (0.03mol), 0.242805g manganese chloride (0.0015mol), 0.108g acrylic acid (0.0015mol), fully dissolved in 30ml deionized water, the above-mentioned mixed solution is placed in a hydrothermal reaction kettle, at 150 ℃ reaction 3h. After cooling, the mixture was washed with deionized water and absolute ethanol until the filtrate was neutral. Grind into powder after drying, take 1g of powder, put it in a beaker containing 100ml of deionized water, add 0.5g of potassium permanganate, stir it on a magnetic stirrer at a speed of 200rpm for 0.5h, and pass the mixture through deionized water After washing to neutrality and drying, manganese dioxide / hydrothermal carbon composite microspheres are obtained.
[0042] (2) Evaluation of catalytic activity
[0043] Get 0.108g benzyl alcohol (0.001mol), 4.6g toluene (0.05mol), the hydrogen peroxide solution (0.0005mol) of 0.057g (30...
Embodiment 3
[0045] (1) Preparation of manganese dioxide / hydrothermal carbon composite microspheres
[0046] Fully dissolve 15g of starch (0.03mol), 4.53g of manganese sulfate (0.03mol), and 1.08g of acrylic acid (0.015mol) in 150ml of deionized water, place the above mixed solution in a hydrothermal reaction kettle, and react at 250°C 24h. After cooling, the mixture was washed with deionized water and absolute ethanol until the filtrate was neutral. Grind into powder after drying, take 1g of powder, put it in a beaker containing 100ml of deionized water, add 10g of potassium permanganate, place it on a magnetic stirrer and stir at a speed of 400rpm for 12h, and wash the mixture with deionized water until Neutral, manganese dioxide / hydrothermal carbon composite microspheres are obtained after drying.
[0047] (2) Evaluation of catalytic activity
[0048] Get 0.108g benzyl alcohol (0.001mol), 13.8g toluene (0.15mol), the hydrogen peroxide solution (0.005mol) of 0.57g (30wt%) mixes with 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com