Preparation method for iron-doped pleated graphene aerogel by high-temperature expansion
A technology of graphene airgel and high-temperature expansion, applied in the direction of graphene, nano-carbon, etc., can solve the problems of high contact resistance and low conductivity, reduce oxidation, increase specific surface area, reduce conductivity and mechanical properties performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
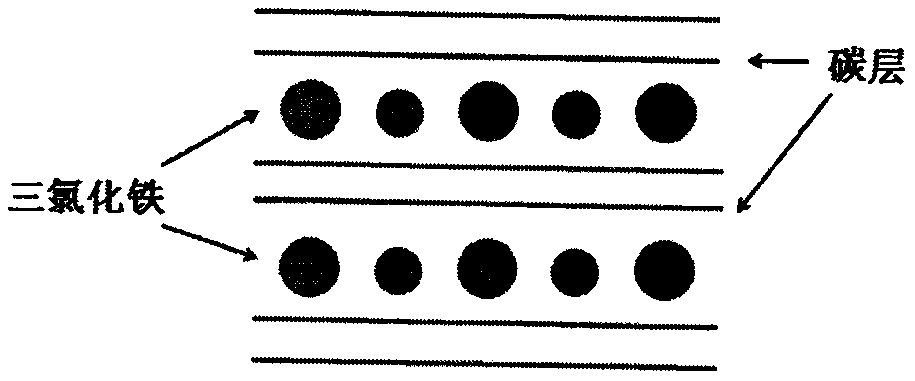
[0028] (1) Mix 300mg of anhydrous ferric chloride and 60mg of expanded graphite evenly, vacuumize, seal in a 50mL glass bottle, and heat at 400°C for 4h to prepare a pure second-order graphite intercalation compound. Dissolve the graphite intercalation compound in Dilute hydrochloric acid solution, filter and dry.
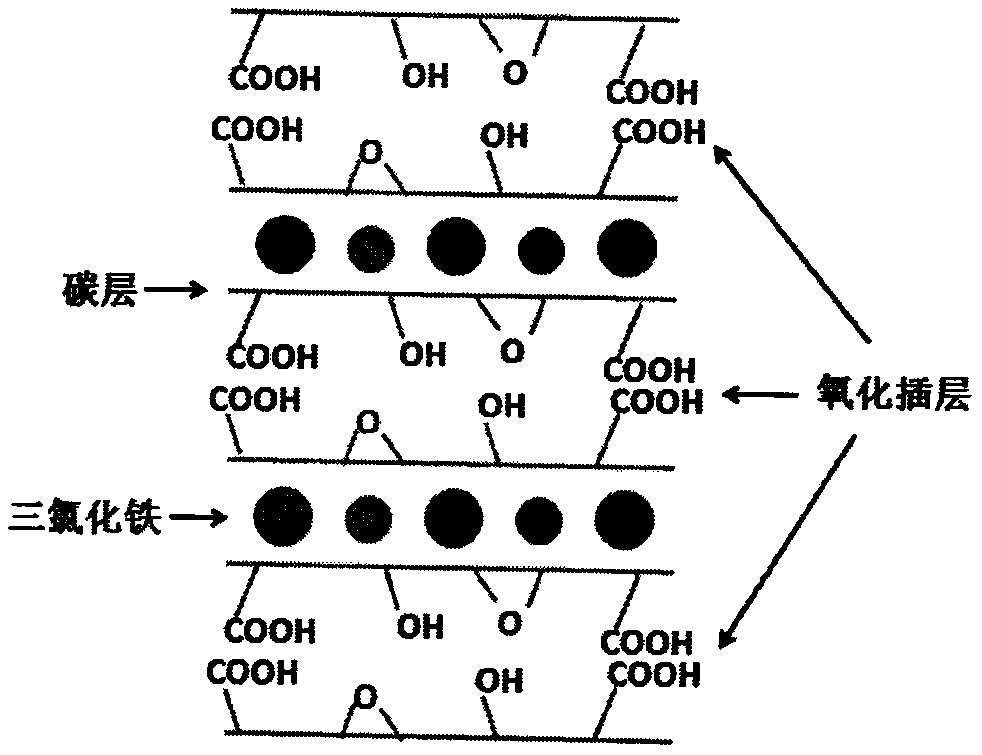
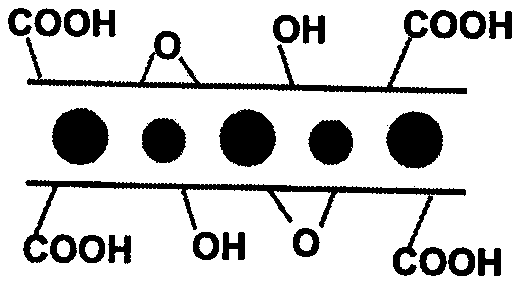
[0029] (2) The graphite intercalation compound was added into a mixed solution of 20 mL of concentrated sulfuric acid and 10 mL of concentrated nitric acid, stirred in ice water (0° C.) for 0.5 hour, 360 mg of sodium chlorate was put into the solution, and stirred at room temperature for 12 hours, The product is centrifuged and cleaned to obtain oxygen-containing group layers and ferric chloride alternately intercalated graphite.
[0030] (3) putting oxygen-containing group layers and ferric chloride intercalated graphite into an aqueous solution for ultrasonic treatment for 1 h to obtain ferric chloride intercalated graphene oxide dispersible in water.
[0031] (...
Embodiment 2
[0034] (1) Mix 300mg of anhydrous ferric chloride and 60mg of expanded graphite evenly, vacuumize, seal in a 50mL glass bottle, and heat at 400°C for 4h to prepare a pure second-order graphite intercalation compound. Dissolve the graphite intercalation compound in Dilute hydrochloric acid solution, filter and dry.
[0035] (2) Add the graphite intercalation compound into 20 mL of concentrated sulfuric acid solution, stir in ice water (0° C.) for 0.5 hours, put 360 mg of potassium permanganate into the solution, stir at room temperature for 12 hours, and centrifuge the product to obtain Oxygen-containing group layers and ferric chloride intercalate graphite alternately.
[0036] (3) putting oxygen-containing group layers and ferric chloride intercalated graphite into an aqueous solution for ultrasonic treatment for 1 h to obtain ferric chloride intercalated graphene oxide dispersible in water.
[0037] (4) Freeze-dry the ferric chloride-intercalated graphene oxide dispersion, ...
Embodiment 3
[0040] (1) Mix 300mg of anhydrous ferric chloride and 60mg of expanded graphite evenly, vacuumize, seal in a 50mL glass bottle, and heat at 400°C for 4h to prepare a pure second-order graphite intercalation compound. Dissolve the graphite intercalation compound in Dilute hydrochloric acid solution, filter and dry.
[0041] (2) The graphite intercalation compound was added into a mixed solution of 20 mL of concentrated sulfuric acid and 10 mL of concentrated nitric acid, stirred in ice water (0° C.) for 0.5 hour, 360 mg of sodium chlorate was put into the solution, and stirred at room temperature for 12 hours, The product is centrifuged and cleaned to obtain oxygen-containing group layers and ferric chloride alternately intercalated graphite.
[0042] (3) putting oxygen-containing group layers and ferric chloride intercalated graphite into an aqueous solution for ultrasonic treatment for 1 h to obtain ferric chloride intercalated graphene oxide dispersible in water.
[0043] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com