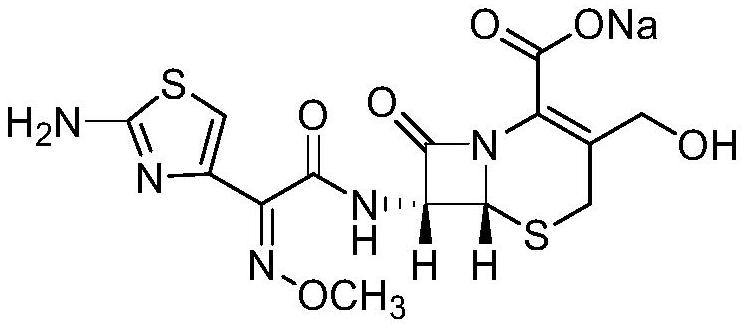
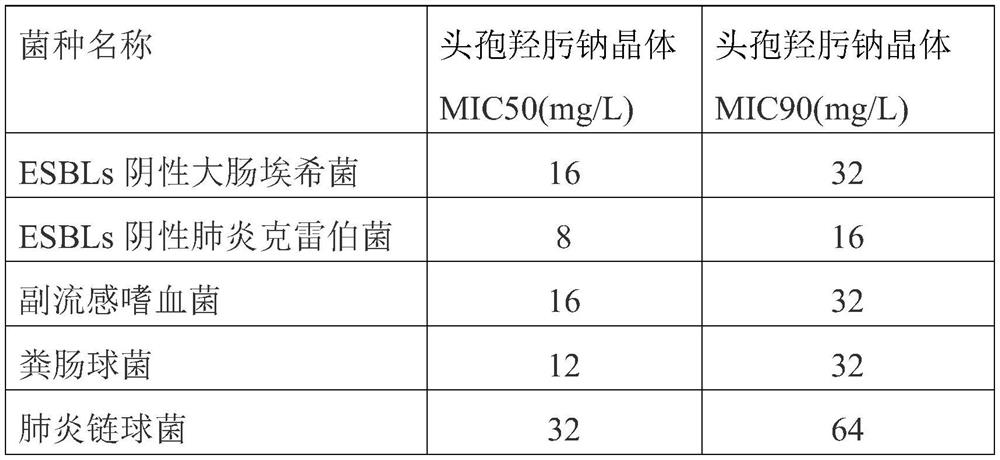
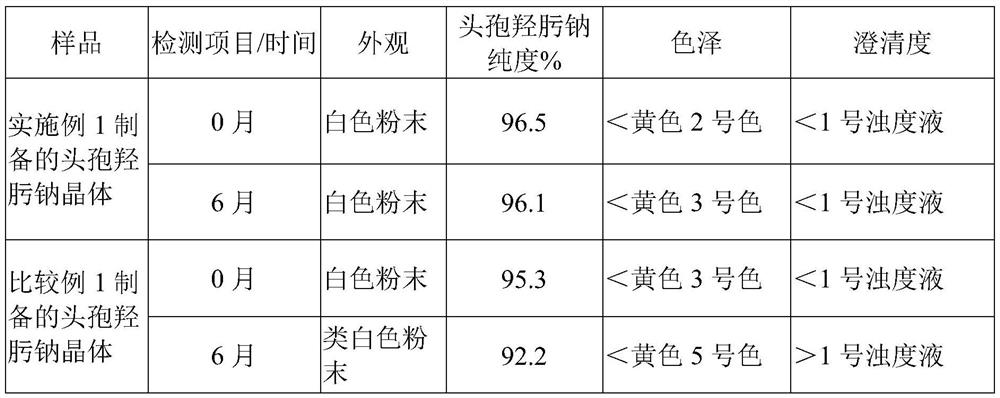
The preparation method of cefhydroxime sodium crystal
A technology of cefadroxime and hydroxymethyl, applied in the field of medicinal chemistry, can solve the problems of reduced antibacterial activity, deepened color, allergic reactions and other problems, and achieves the effects of reducing the generation of impurities, stable and uniform crystal form, and mild and controllable conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 3.6 grams of sodium acetate trihydrate into 14.4 ml of water, stir and dissolve, and prepare an aqueous sodium acetate solution of about 20 wt %;
[0028] At 15-20°C, add 20ml of ethyl acetate and 0.2ml of caprylic acid glyceride into the reaction vessel, stir well, add 10ml of water under stirring, and control the stirring speed at 60-70 rpm to form a uniform emulsion Then, 10 g of cefhydroxamic acid and the above-mentioned about 20 wt % sodium acetate aqueous solution are added to the emulsion in batches at the same time, so that the pH value remains between 6 and 8 for a salt-forming reaction, and the addition is stirred for 30 minutes;
[0029] After the salt-forming reaction, transfer to a centrifuge, centrifuge at a centrifugal speed of 15,000-20,000r / min to break the emulsion, then let it stand and separate the layers, remove the ethyl acetate layer, add 1g of activated carbon to the obtained water layer, and stir for 10 minutes , filtered, after the activate...
Embodiment 2
[0042] Add 3.3 grams of sodium acetate trihydrate into 10 ml of water, stir and dissolve, and prepare an aqueous sodium acetate solution of about 25 wt %;
[0043] At 25-30°C, add 15ml of ethyl acetate and 0.3ml of caprylic acid glyceride into the reaction vessel, stir well, then add 10ml of water while stirring, and control the stirring speed at 90-100 rpm to form a uniform emulsion ; Then 10g of cefhydroxamic acid and the above-mentioned about 25wt% sodium acetate aqueous solution are added to the emulsion in batches at the same time, so that the pH value remains between 6 and 8 for a salt-forming reaction, and the addition is completed and stirred for 25 minutes;
[0044] After the salt-forming reaction, transfer to a centrifuge, centrifuge at a centrifugal speed of 20,000-25,000r / min to break the emulsification, then let it stand and separate the layers, remove the ethyl acetate layer, add 2g of activated carbon to the obtained water layer, and stir for 10 minutes , filter...
Embodiment 3
[0047] Add 3.95 grams of sodium acetate trihydrate into 19.8 ml of water, stir and dissolve, and prepare an aqueous sodium acetate solution of about 16.6 wt %;
[0048] At 5-10°C, add 30ml of ethyl acetate and 1.5ml of caprylic acid glyceride into the reaction vessel, stir well, add 30ml of water under stirring, and control the stirring speed at 50-65 rpm to form a uniform emulsion ; Then 10g of cefhydroxamic acid and the above-mentioned about 16.6wt% sodium acetate aqueous solution are added to the emulsion in batches at the same time, so that the pH value remains between 6 and 8 for a salt-forming reaction, and the addition is stirred for 30 minutes;
[0049] After the salt-forming reaction, transfer to a centrifuge, centrifuge at a centrifugal speed of 15,000-20,000r / min to break the emulsification, then let it stand and separate the layers, remove the ethyl acetate layer, add 5g of activated carbon to the obtained water layer, and stir for 10 minutes , filtered, after the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com