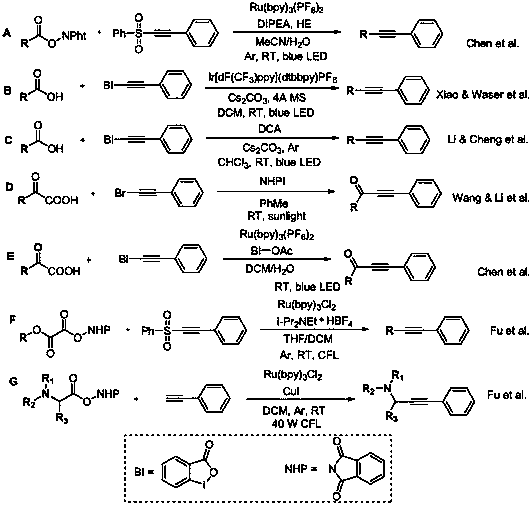
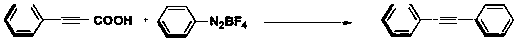Preparation method of aryl alkyne catalyzed by visible light
A technology of aryl alkynes and visible light, which is applied in the field of preparation of aryl alkynes, can solve problems such as toxicity, high cost efficiency, and limited application, and achieve the effects of mild reaction conditions, simple catalysts, and high reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Under the protection of nitrogen, add phenylpropylic acid (0.5mmol), diazonium phenylfluoroborate (0.5mmol), additive (0.6mmol), photocatalyst (1mol%), reaction solvent (1mL) in reaction tube, The reaction was carried out under the action of a visible light source (3.0W) at 25°C, and the reaction was monitored by TLC. After 12 hours of reaction, it was filtered, the solvent was removed by spin, and the target product was obtained. The reaction conditions and results are shown in Table 1.
[0041] The reaction formula is as follows:
[0042]
[0043] Table 1 Reaction conditions and reaction results of Example 1
[0044]
[0045]
Embodiment 2
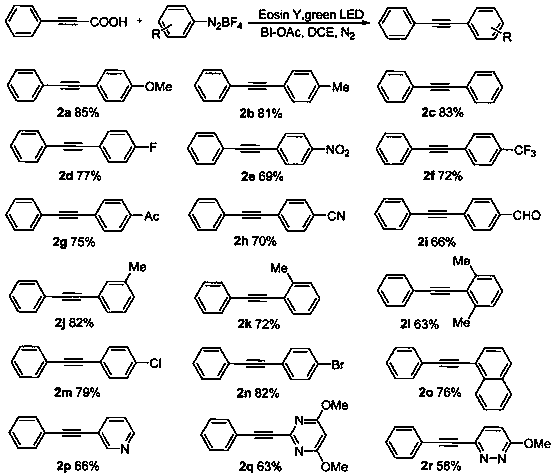
[0047] Under nitrogen protection, add phenylpropylic acid (0.5mmol), aryl fluoroborate diazonium salt (0.5mmol), additive BI-OAc (0.6mmol), photocatalyst Eosin Y (1mol%) in reaction tube, react The solvent DCE (1mL) was reacted at 25°C under the action of a visible light source green LED (3.0W), and the reaction was monitored by TLC. After 12 hours of reaction, it was filtered, the solvent was removed, and the target product was obtained by column chromatography.
[0048] The reaction formula and reaction result are as follows:
[0049]
Embodiment 3
[0051] Under nitrogen protection, add arylpropiolic acid (0.5mmol), phenylfluoroborate diazonium salt (0.5mmol), additive BI-OAc (0.6mmol), photocatalyst Eosin Y (1mol%) in reaction tube, The reaction solvent DCE (1mL) was reacted at 25°C under the action of a visible light source green LED lamp (3.0W), and the reaction was monitored by TLC. After 12 hours of reaction, it was filtered, the solvent was removed, and the target product was obtained by column chromatography.
[0052] The reaction formula and reaction result are as follows:
[0053]
[0054] The characterization data of some products are as follows:
[0055] 1-Methoxy-4-(phenylethynyl)benzene(2a,3a)
[0056] White solid (88mg, 85%). 1 H NMR (CDCl 3 ,400MHz):δ7.52(dt,J=4.0,2.0Hz,2H),7.49–7.45(m,2H),7.36–7.28(m,3H),6.88(d,J=8.8Hz, 2H), 3.84(s,3H). 13 C NMR (CDCl 3 ,100MHz):δ159.5,133.1,131.6,128.3, 127.8,123.6,115.3,114.0,89.5,88.1,55.2.HRMS(EI)Calcd for C 15 h 13 O [M+H] + , 209.0961; found, 209.0970.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com