A long-term stable supercooled phase change heat storage material and its preparation method and application
A phase change heat storage material and supercooling technology, applied in the field of phase change materials, can solve the problems of spontaneous crystallization and heat release, and achieve the effects of reducing solid-liquid interface energy, high heat storage, and avoiding self-crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This example is the preparation of a long-term stable supercooled phase change energy storage material.
[0045] (1) get 92 parts of hydrated salt, wherein the ratio of sodium thiosulfate pentahydrate and sodium acetate trihydrate is 90:10; 6 parts of liquid hydrocarbons are Span 80; 2 parts of thickener, Sodium carboxymethyl cellulose.
[0046] (2) Physically blend Span 80, sodium carboxymethylcellulose sodium thiosulfate pentahydrate and sodium acetate trihydrate, heat and melt in a constant temperature water bath at 65°C, stir with a magnetic rotor at a speed of 200rpm while heating The mixture was left for 15 min to obtain a molten mixture.
[0047] (3) The molten mixture prepared in step (2) was dried in a vacuum oven at 40° C. for 30 minutes, and then ultrasonically treated for 20 minutes to obtain a long-term stable supercooled phase change heat storage material.
Embodiment 2
[0049] This example is the preparation of a long-term stable supercooled phase change energy storage material.
[0050] (1) get 90 parts of hydrated salt, wherein the ratio of sodium thiosulfate pentahydrate and sodium acetate trihydrate is 90:10; 8 parts of liquid hydrocarbons are Tween 80; 2 parts of thickener, for sodium alginate.
[0051](2) Physically blend Tween 80, sodium alginate pentahydrate, sodium thiosulfate pentahydrate, and sodium acetate trihydrate, heat and melt in a constant temperature water bath at 70°C, and stir the mixture with a magnetic rotor at a speed of 200 rpm for 15 minutes while heating , to obtain a molten mixture.
[0052] (3) The molten mixture prepared in step (2) was dried in a vacuum oven at 40° C. for 30 minutes, and then ultrasonically treated for 20 minutes to obtain a long-term stable supercooled phase change heat storage material.
Embodiment 3
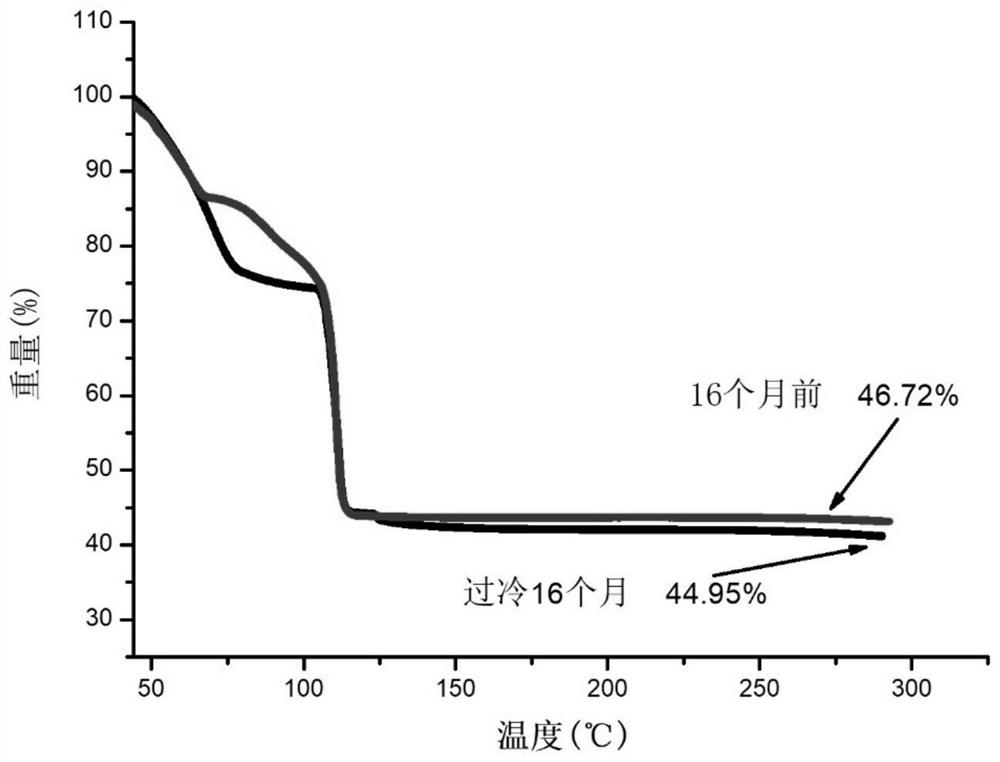
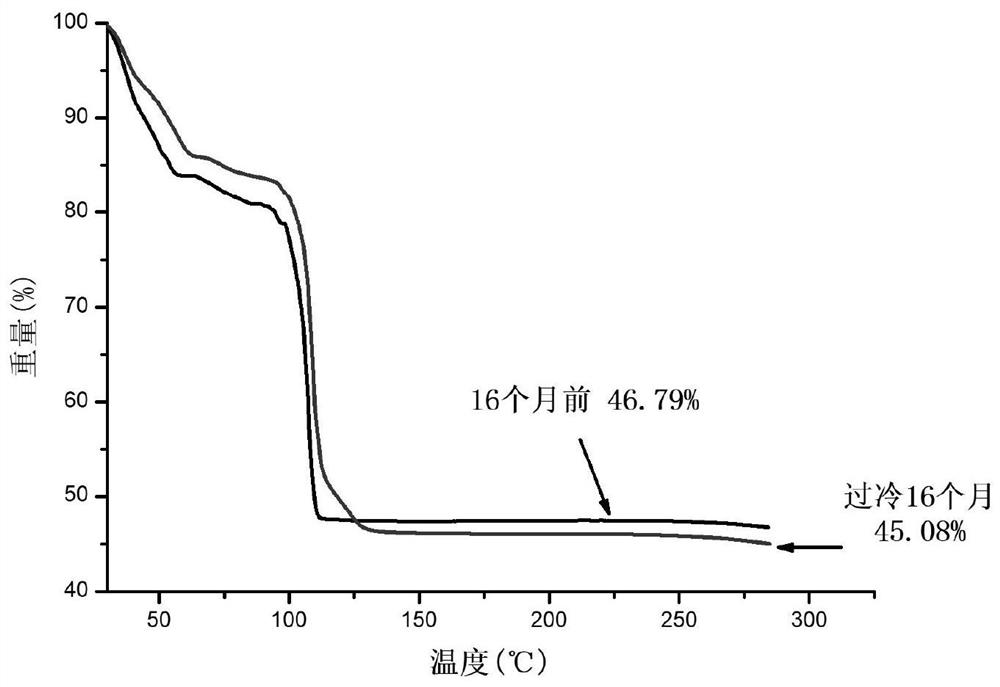
[0064] The long-term stable supercooled phase change energy storage material prepared in Example 1-2 and Comparative Example 1-2 was placed at a temperature of 5°C, and the time difference in the stable supercooling time at a low temperature was observed.
[0065] The phase-change energy storage material provided in Example 1 can be stably undercooled at 5°C for 16 months, and the crystallization exotherm is normal, the latent heat of phase change is 182.7J / g, and the undercooling degree is as high as 43°C.
[0066] The long-term stable supercooled phase change energy storage material provided in Example 2 can be stably supercooled for 16 months at a temperature of 5°C, and the crystallization exotherm is normal, the latent heat of phase change is 180.1J / g, and the supercooling degree during this period is as high as 43°C .
[0067] The phase-change energy storage material provided in Comparative Example 1 began to crystallize after being maintained at a temperature of 5° C. f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com