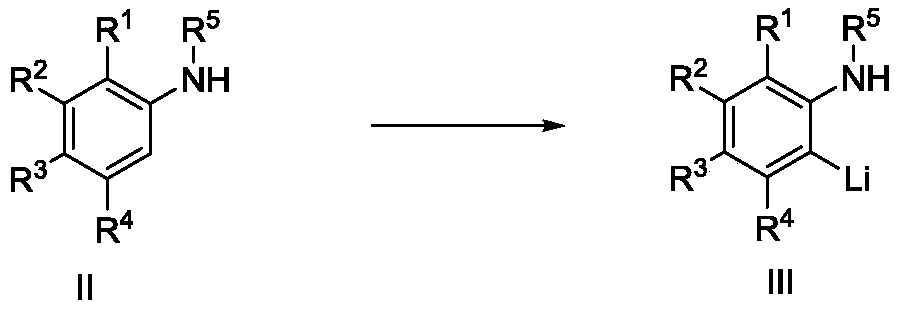
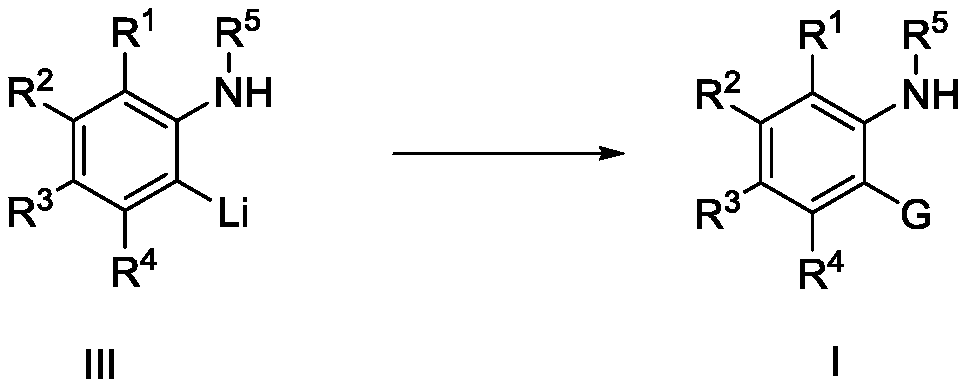
Preparation method of arylamine o-substituted compound
A technology of compound and metal lithium compound, applied in the preparation of organic compounds, lithium organic compounds, carboxylic acid amides, etc., can solve the problem of single preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
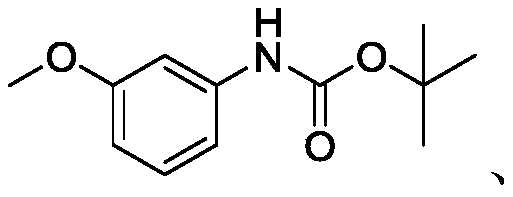
[0075] N-Boc-3-methoxyaniline (compound A) (223 g, 1 mol) was dissolved in THF to form a 0.5 mol / L solution 1, and 2.5 M n-BuLi n-hexane as solution 2. Then solution 1 and solution 2 were pumped into the first three-way connection of the tubular reactor at a flow rate of 60mL / min (0.03mol / min) and 30mL / min (0.075mol / min) to start mixing, and the reaction was carried out at 0°C. After staying for 14s, the reaction stays at 45°C for 1.3min, and trimethyl borate is pumped into the second tee connection at a flow rate of 10mL / min (0.09mol / min) to start mixing. After the reaction stays at 45°C for 2.1min, the The outflow from the reactor was quenched dropwise into a stirred flask containing 1000 g of water. Acidify with 6N hydrochloric acid to pH=2, stir for 30min, add 500g of ethyl acetate, separate the water layer, wash the organic layer twice with 1000g of water, then concentrate under reduced pressure at 40°C to the remaining 400g, add 600g of n-hexane, Stir at 5-1...
Embodiment 2
[0077] N-Boc-3-methoxyaniline (compound A) (223 g, 1 mol) was dissolved in THF to form a 0.5 mol / L solution 1, and 2.5 M n-BuLi n-hexane as solution 2. Then solution 1 and solution 2 were pumped into the first three-way connection of the tubular reactor at a flow rate of 60ml / min (0.03mol / min) and 30mL / min (0.075mol / min) to start mixing, -15°C After the reaction stays for 5 minutes, trimethyl borate is pumped into the second three-way connection at a flow rate of 10mL / min (0.09mol / min) to start mixing. Quench with a stirred flask containing 1000 g of water. Acidify with 6N hydrochloric acid to pH=2, stir for 30min, add 500g of ethyl acetate, separate the water layer, wash the organic layer twice with 1000g of water, then concentrate under reduced pressure at 40°C to the remaining 400g, add 600g of n-hexane, Stir at 5-10°C for 30 minutes and filter, then wash the filter cake with 200g of n-hexane, and dry under reduced pressure at 40°C to constant weight to obtain 238g of comp...
Embodiment 3
[0079] N-Boc-3-methoxyaniline (compound A) (223 g, 1 mol) was dissolved in THF to form a 0.5 mol / L solution 1, and 2.5 M n-BuLi n-hexane as solution 2. Then solution 1 and solution 2 were pumped into the first three-way connection of the tubular reactor at a flow rate of 60mL / min (0.03mol / min) and 30mL / min (0.075mol / min) to start mixing, and the reaction was carried out at 60°C. After staying for 0.3min, trimethyl borate was pumped into the second three-way connection at a flow rate of 10.0mL / min (0.09mol / min) to start mixing. After staying at 60°C for 0.5min, it flowed out from the tubular reactor and dropped to Quenched in a stirred flask with 1000 g of water. Acidify with 6N hydrochloric acid to pH=2, stir for 30min, add 500g of ethyl acetate, separate the water layer, wash the organic layer twice with 1000g of water, then concentrate under reduced pressure at 40°C to the remaining 400g, add 600g of n-hexane, Stir at 5-10°C for 30 minutes and filter, then wash the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com