A kind of preparation method of using iron-containing solid waste to prepare vocs catalyst
A technology of catalyst and catalyst carrier, which is applied in the field of air pollution control, can solve problems such as the blank period of research, achieve broad market application prospects, good low-temperature catalytic effect, and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
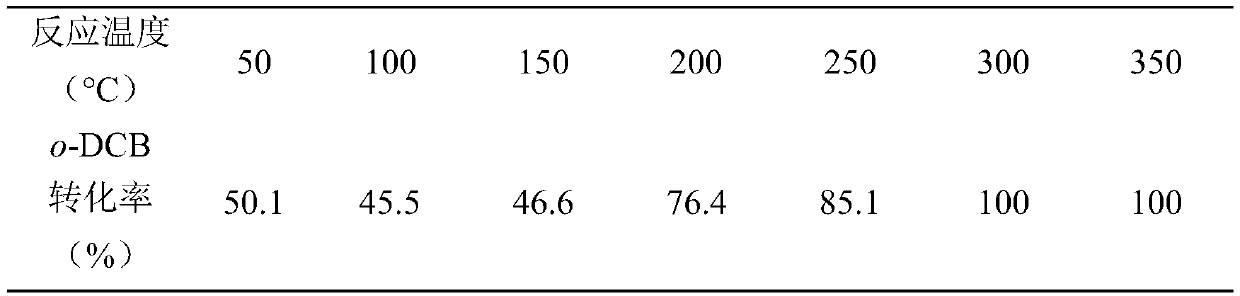
[0032] (1) Prepare catalyst carrier with iron-containing solid waste: take 200g iron-containing solid waste (wherein SiO 2 : 40%; Fe 2 o 3 : 11%; TiO 2 MgO: 8%; MgO: 10%; Others: 31%) and organic matter 6g (corn stalk); after the two are pulverized with a pulverizer, the powder between 180~300 meshes is obtained through sieving, and the two powders are mixed evenly. Then, it was calcined at 500°C for 2 h under nitrogen atmosphere to obtain the catalyst support, denoted as TC.
[0033] (2) Catalyst preparation: take 1g catalyst carrier and be dispersed in the mixed solution that contains manganese nitrate and cerium nitrate, wherein the mass ratio of Mn element and Mn element and catalyst carrier total mass is 0.15, and the mol ratio of metal Ce element and Mn element is 0.3. Stir thoroughly for 30 min, then transfer the obtained mixture to a 90°C water bath environment and continue stirring until the water evaporates completely. The evaporated solid was fully dried at 130...
Embodiment 2
[0045] (1) Prepare catalyst carrier with iron-containing solid waste: take 250g iron-containing solid waste (wherein SiO 2 : 37%; Fe 2 o 3 : 9%; TiO 2 MgO: 5%; MgO: 12%; Others: 37%) and organic matter 7.5g (corn cob), after the two are pulverized with a pulverizer, the powder between 180~250 meshes is obtained through sieving, and then 450 under nitrogen atmosphere Calcined at ℃ for 2h to obtain a catalyst carrier, denoted as TC.
[0046] (2) Catalyst preparation: Weigh 1g of the catalyst carrier and disperse it in a mixed solution containing cerium nitrate (active component) and cobalt nitrate (auxiliary), wherein the mass ratio of the Ce element to the total mass of the Ce element and the catalyst carrier is 0.15, and the metal The molar ratio of the Co element to the Ce element was 0.45. Stir thoroughly for 30 min, then transfer the obtained mixture to an 80°C water bath environment and continue stirring until the water evaporates completely. The evaporated solid was ...
Embodiment 3
[0054] (1) Prepare catalyst carrier with iron-containing solid waste: take 200g iron-containing solid waste (wherein SiO 2 : 32%; Fe 2 o 3 : 6%; TiO 2 MgO: 5%; MgO: 4%; Others: 53%) and organic matter 8g (corncobs), after the two are pulverized with a pulverizer, get the powder between 180~300 mesh through sieving. Then, it was calcined at 400°C for 3 h under nitrogen atmosphere to obtain the catalyst support, denoted as TC.
[0055] (2) Catalyst preparation: Weigh 1g carrier and disperse it in a mixed solution containing nickel nitrate and cerium nitrate, wherein the mass ratio of Ni element to the total mass of Ni element and catalyst carrier is 0.15, and the molar ratio of metal Ce element to Ni element is 0.15 . Stir thoroughly for 60 min, then transfer the obtained mixture to a 90°C water bath environment and continue stirring until the water evaporates completely. The evaporated solid was fully dried at 130°C for 5 hours, then ground into a uniform powder, and final...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com