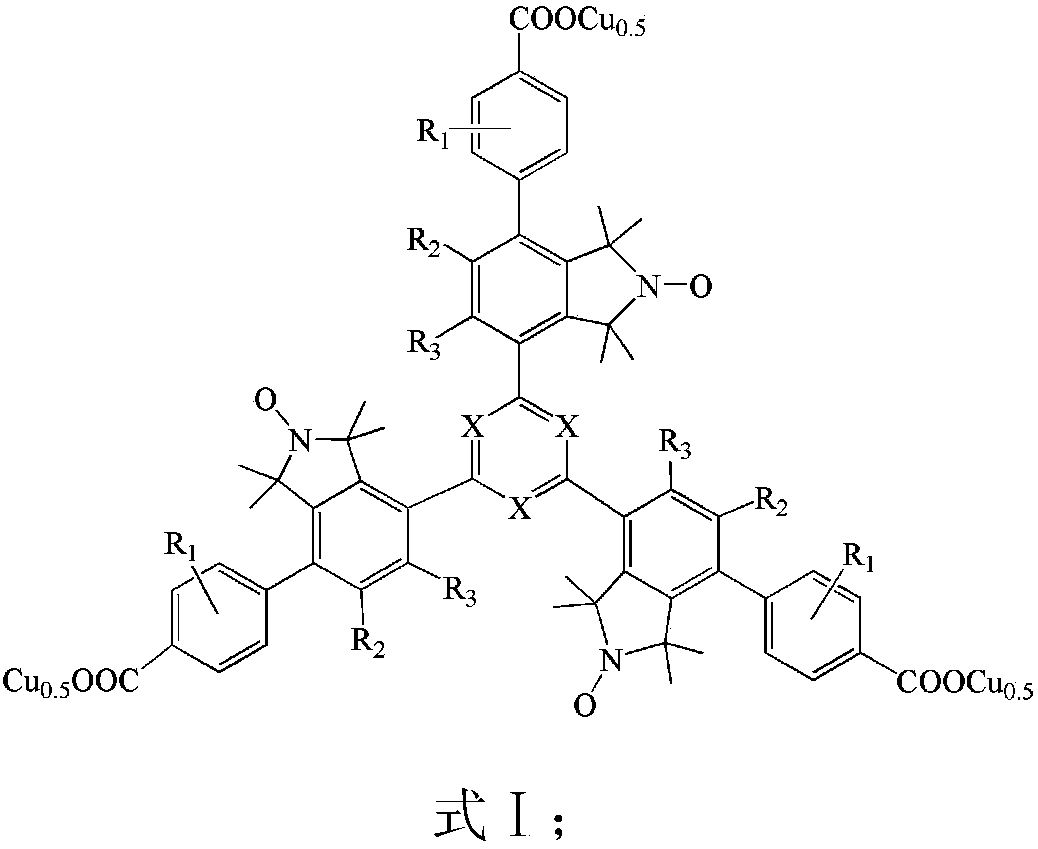
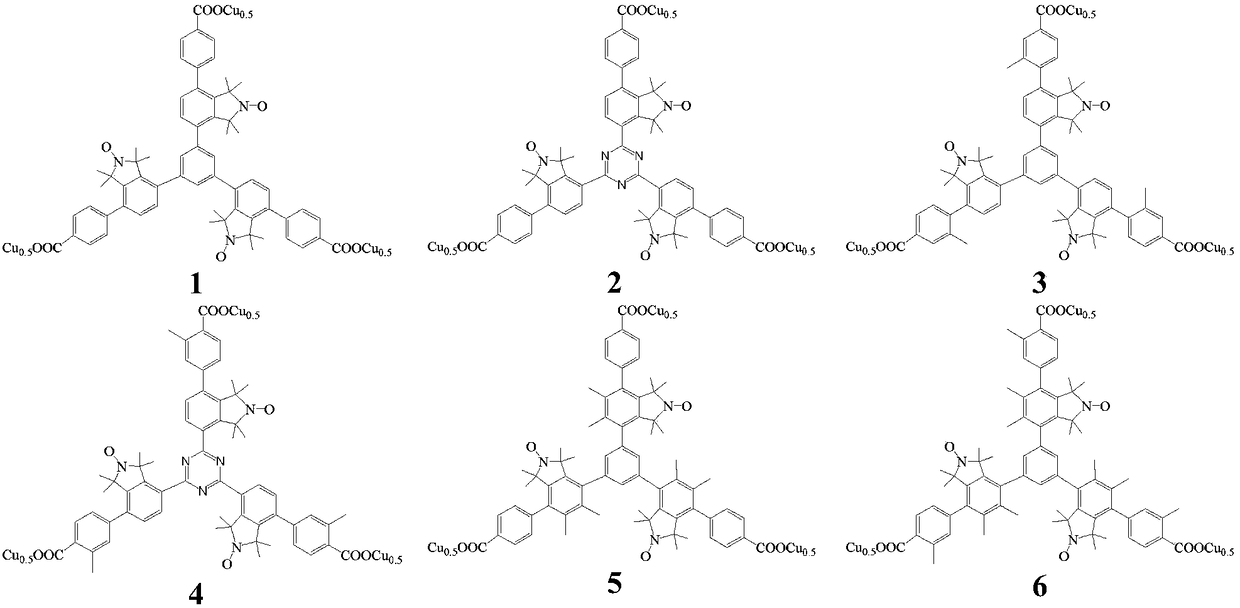
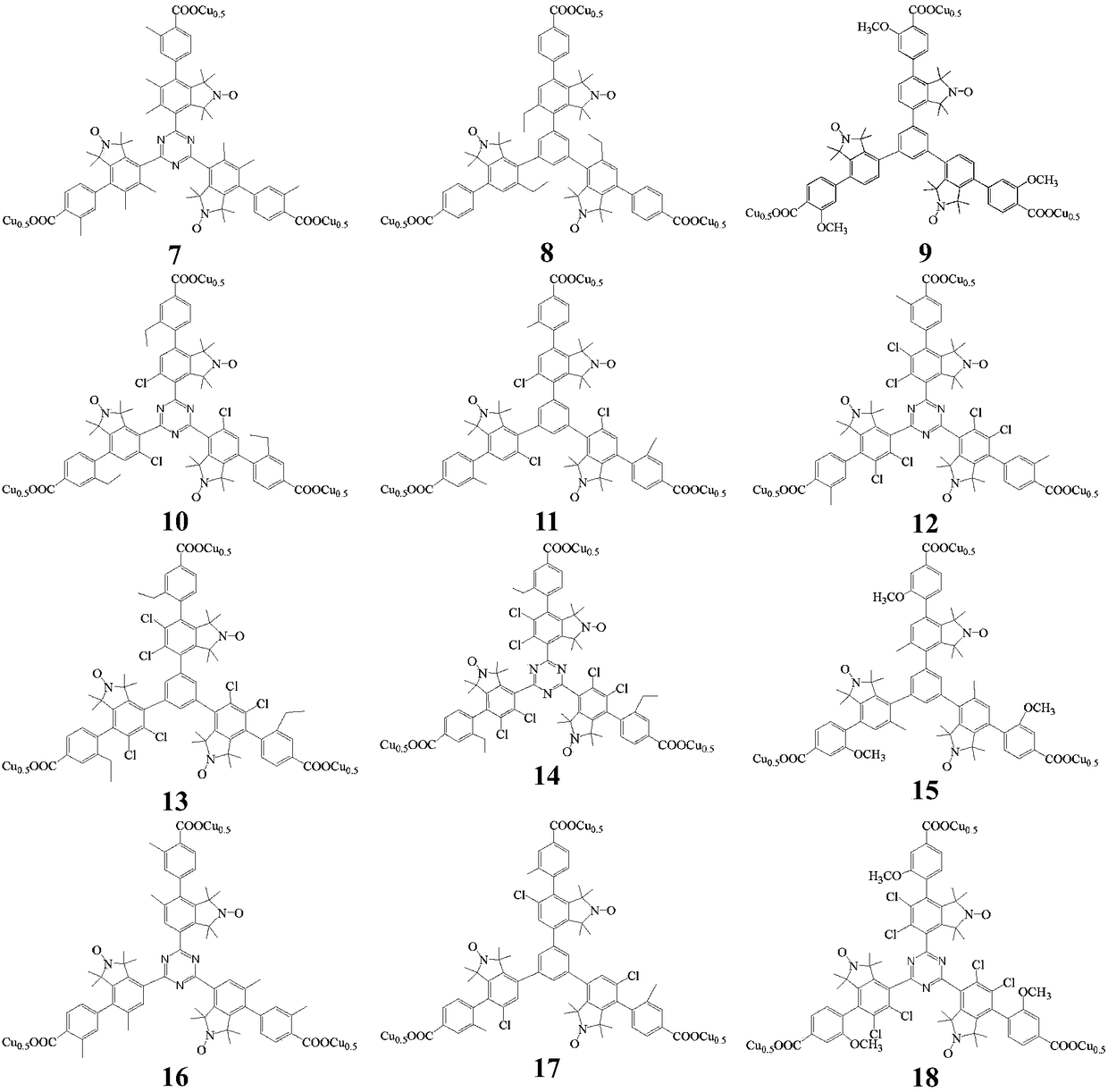
Mesitylene biaryl (triazine) tricarboxylic acid copper complex containing nitrogen oxygen radicals, and application thereof in preparing menadione
A technology of nitroxide radicals and terary arylbenzenes, which is applied in the preparation of quinone oxides, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problem that metal salt catalysts cannot be recycled, recycled, produced high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Preparation of nitrogen-oxygen free radical-s-triarylbenzene (triazine) bromide (20)
[0068] Under the protection of an inert gas (high-purity nitrogen), add 3.3 mmol of nitrogen-containing free radical dibromide (19), 1 mmol of triphenylboronic acid, 0.15 mmol of tetrakistriphenylphosphine palladium, and 6 mmol of carbonic acid to a 50 ml Schlek reaction tube. Potassium and 25ml of toluene water solvent (the volume ratio of toluene to water is 3:1), replace the reaction tube with nitrogen for 3 times, then heat to 90°C in an oil bath under magnetic stirring, and reflux for 24h; remove the oil bath, and react The solution was concentrated with a rotary evaporator, and the residue was separated with ethyl acetate as a developing solvent by thin-layer chromatography on silica gel to obtain a pure product containing nitrogen-oxygen radical-containing triarylbenzene (triazine) bromide (20), with a yield of 72%. . Mass spectrum (ESI) MS of the product: m / z: 877.1 (M+H)...
Embodiment 2
[0074] (1) Preparation of nitrogen-oxygen free radical-s-triarylbenzene (triazine) bromide (24)
[0075] Under the protection of an inert gas (high-purity nitrogen), add 4 mmol of nitrogen-containing free radical dibromide (23), 1 mmol of s-triazine boronic acid, 0.12 mmol of tetrakistriphenylphosphine palladium, and 6 mmol of cesium carbonate to a 50 ml Schlek reaction tube and 25ml of toluene water solvent (the volume ratio of toluene to water is 3:1), replace the reaction tube with nitrogen for 3 times, then heat to 90°C with an oil bath under magnetic stirring, and reflux the reaction for 20h, remove the oil bath, and the reaction solution Concentrate with a rotary evaporator, use ethyl acetate as a developing solvent for the residue, and separate with silica gel thin-layer chromatography to obtain a pure product containing nitrogen-oxygen radical-containing triarylbenzene (triazine) bromide (24), with a yield of 62%. Mass spectrum (ESI) MS of the product: m / z: 1069.8 (M-H...
Embodiment 3
[0081] (1) Preparation of nitroxy free radical-s-triarylbenzene ((triazine)) bromide (25)
[0082] The method is similar to Example 1, and the nitroxide-containing free radical-s-triarylbenzene ((triazine)) bromide (25) is prepared;
[0083] (2) Preparation of tertiary arylbenzene ((triazine)) tricarboxylic acid (26) containing nitrogen and oxygen free radicals
[0084] Under the protection of inert gas (high-purity nitrogen), in the Schlek reaction tube of 50ml, add the nitroxide-containing free radical s-triarylbenzene ((triazine)) bromide (25), 5mmol 4 that 1mmol step (1) makes - p-carboxyphenylboronic acid, 0.12mmol palladium chloride, 0.15mmol 2'-dicyclohexyl-2,6-dimethoxy-3-sulfonic acid-1,1'-biphenyl sodium salt, 6mmol sodium carbonate and 30ml Replace the reaction tube with nitrogen for 3 times, then heat to 100°C with an oil bath under magnetic stirring, and reflux for 32 hours; remove the oil bath, filter after the reaction, acidify the filtrate, adjust the pH to 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com