A kind of preparation method of catalyst for caprolactam amination
A technology of caprolactam and catalyst, applied in the direction of catalyst activation/preparation, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of low conversion rate, low reaction temperature, low selectivity, etc., and achieve low cost and simple preparation , Preparation and easy-to-obtain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
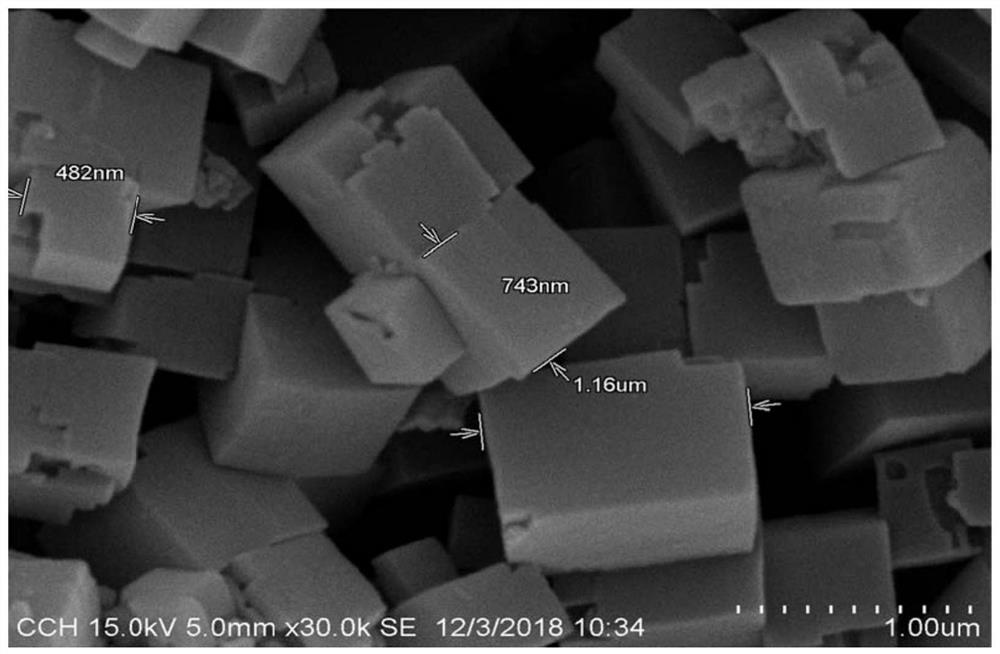
[0024] Weigh 5g of magnesium nitrate, 5g of aluminum nitrate, 1g of nickel nitrate and 150g of water to prepare a mixed solution, weigh 100g of phospho-aluminum molecular sieve, and add the aqueous solution of the active component to the phospho-aluminum molecular sieve; the rotary evaporator is heated to 60° C. After 4 hours, the water was drained under reduced pressure; the filter cake was dried at 120 °C, formed, and calcined at 550 °C for 6 hours to obtain the catalyst (see the morphology and specific surface area). Figure 1-3 ). 4 g of the calcined catalyst was loaded into a fixed bed, and the temperature was raised to 420° C. The caprolactam was dissolved in acetonitrile and then fed at a space velocity of 0.5h-1. The reaction solution was cooled and collected, and the sampling was controlled. The conversion rate of the raw material caprolactam was 81%, and the product The selectivity of 6-aminocapronitrile is greater than 99.0%.
Embodiment 2
[0026] Weigh 7g of magnesium nitrate, 7g of aluminum nitrate, 1g of nickel nitrate and 170g of water to prepare a mixed solution, weigh 100g of phospho-aluminum molecular sieve, and add the aqueous solution of the active component to the phospho-aluminum molecular sieve; the rotary evaporator is heated to 65 ° C, rotate After 4 hours, the water was drained under reduced pressure; the filter cake was dried at 120 °C, formed, and calcined at 550 °C for 5 hours to obtain the catalyst. 4 g of the calcined catalyst was loaded into a fixed bed, heated to 450 °C, and the caprolactam was dissolved in acetonitrile and fed at a space velocity of 0.5 h-1. The reaction liquid was cooled and collected, and the sampling was controlled. The conversion rate of the raw materials was 82.2%, and the selectivity greater than 99.0%.
Embodiment 3
[0028] Weigh 9g of magnesium nitrate, 9g of aluminum nitrate, 2g of nickel nitrate and 200g of water to prepare a mixed solution, weigh 100g of phospho-aluminum molecular sieve, and add the aqueous solution of the active component into the phospho-aluminum molecular sieve; the rotary evaporator is heated to 65° C. After 4 hours, the water was drained under reduced pressure; the filter cake was dried at 120°C, formed, and calcined at 550°C for 6 hours to obtain the catalyst. 4 g of the calcined catalyst was loaded into a fixed bed, heated to 420°C, and the caprolactam was dissolved in acetonitrile and fed at a space velocity of 0.5h-1. The reaction solution was cooled and collected, and the sampling was controlled. The conversion rate of the raw materials was 82.2%, and the selectivity greater than 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com