Cyanostilbene fluorescence epoxy compound, and preparation method and application thereof
A cyanostilbene, oxygen compound technology, applied in chemical instruments and methods, epoxy resin glue, epoxy resin coating and other directions, can solve problems such as single type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] One-pot Preparation of Cyanostilbene Fluorescent Epoxy Compound C-1
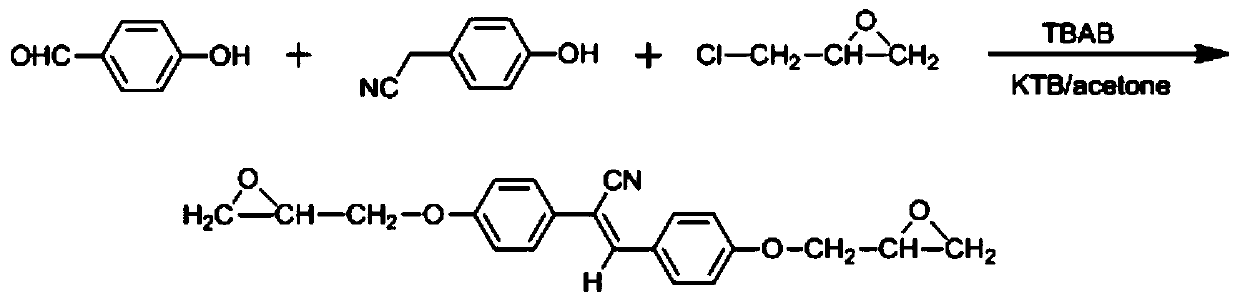
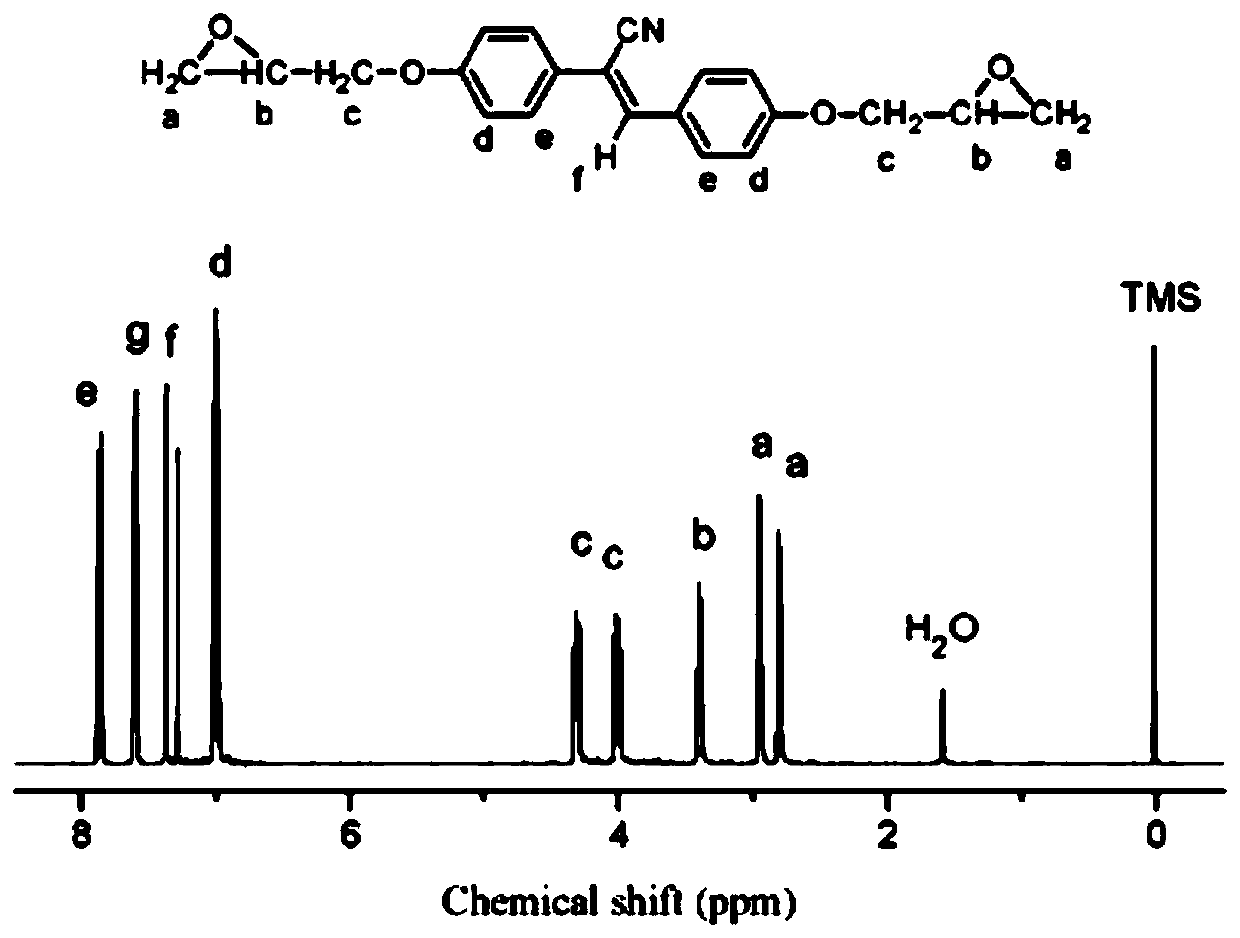
[0063] Add p-hydroxybenzaldehyde (0.01mol, 1.22g), p-hydroxybenzonitrile (0.01mol, 1.33g) and epichlorohydrin (0.10mol, 9.25g) into a 100mL single-necked bottle in turn, and heat up to 68°C. After the raw materials are dissolved, add tetrabutylammonium bromide (0.001mol, 0.322g), react for 4 to 6 hours, add 100mL of ethanol, stir for 10 minutes, then add potassium tert-butoxide (0.01mol, 1.12g), continue The reaction was stopped for 4 hours. After the temperature of the reaction system dropped to room temperature, the solid product was collected by suction filtration to obtain a crude product, which was recrystallized with 80 mL of ethanol to obtain 2.23 g of a pure product with a yield of 64%.
Embodiment 2
[0065] The Step-by-Step Preparation of Cyanostilbene Fluorescent Epoxy Compound C-1
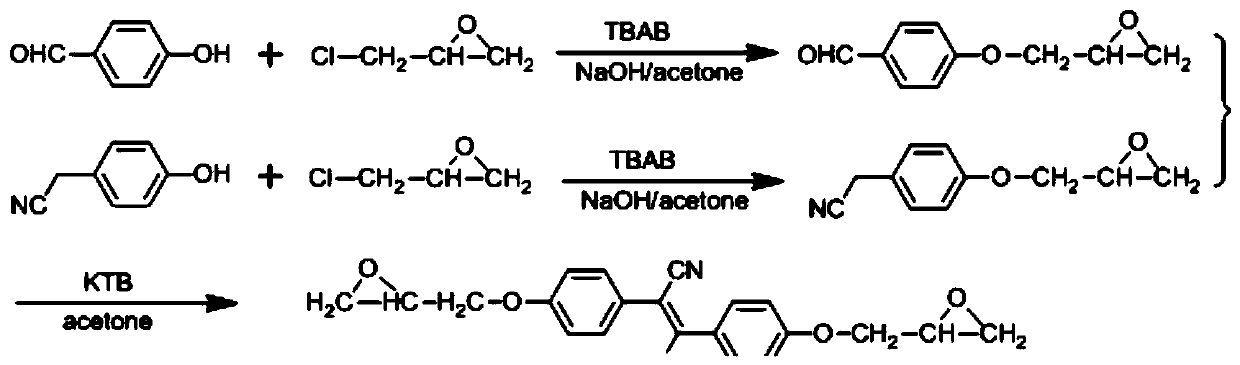
[0066] (1) Preparation of 4-(2-oxirane methoxy) benzaldehyde
[0067] Add p-hydroxybenzaldehyde (0.01mol, 1.22g) and epichlorohydrin (0.10mol, 9.25g) into a 100mL single-necked bottle with a magnet, raise the temperature to 68°C, and add tetrabutyl bromide after the raw materials are dissolved Ammonium chloride (0.001mol, 0.322g), reacted for 6 hours, and then added 15% aqueous sodium hydroxide solution (0.02mol, 0.80g), and continued to react for 3 hours, then stopped the reaction. Add 60 mL of toluene and stir for 10 minutes, release the lower water layer with a separatory funnel, wash the organic phase twice with water, collect the organic phase layer and spin dry the solvent to obtain the crude product, which is separated by column with dichloromethane as the eluent, 1.6 g of pure product was obtained, yield 89%.
[0068] (2) Preparation of 4-(2-oxirane methoxy) benzyl nitrile
[0069]...
Embodiment 3
[0073] One-pot Preparation of Cyanostilbene Fluorescent Epoxy Compound C-2
[0074]Add p-hydroxybenzaldehyde (0.01mol, 1.22g) and epichlorohydrin (0.10mol, 9.25g) into a 250mL single-necked bottle with a magnet, heat up to 68°C, and add tetrabutyl Ammonium bromide (0.001mol, 0.322g), after reacting for 8 hours, add terephthalonitrile (0.0045mol, 0.7000g) and 150mL of ethanol, after stirring for 10 minutes, add solid sodium hydroxide (0.03mol, 1.20g) , Stop the reaction after continuing to react for 4 to 6 hours. After the temperature of the reaction system dropped to room temperature, the solid product was collected by suction filtration, washed with water, and dried to obtain the crude product, which was recrystallized with a mixed solvent of DMF and ethanol (V:V=3:1) to obtain the yellow pure product 1.95 g, yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com