Preparation method and application of photothermal double stimulation response hydrogel containing alpha-cyclodextrin
A dual-stimuli-response, cyclodextrin technology is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulations. It can solve the problem of insufficient loading of drug molecules and the ineffective release of drug molecules to environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
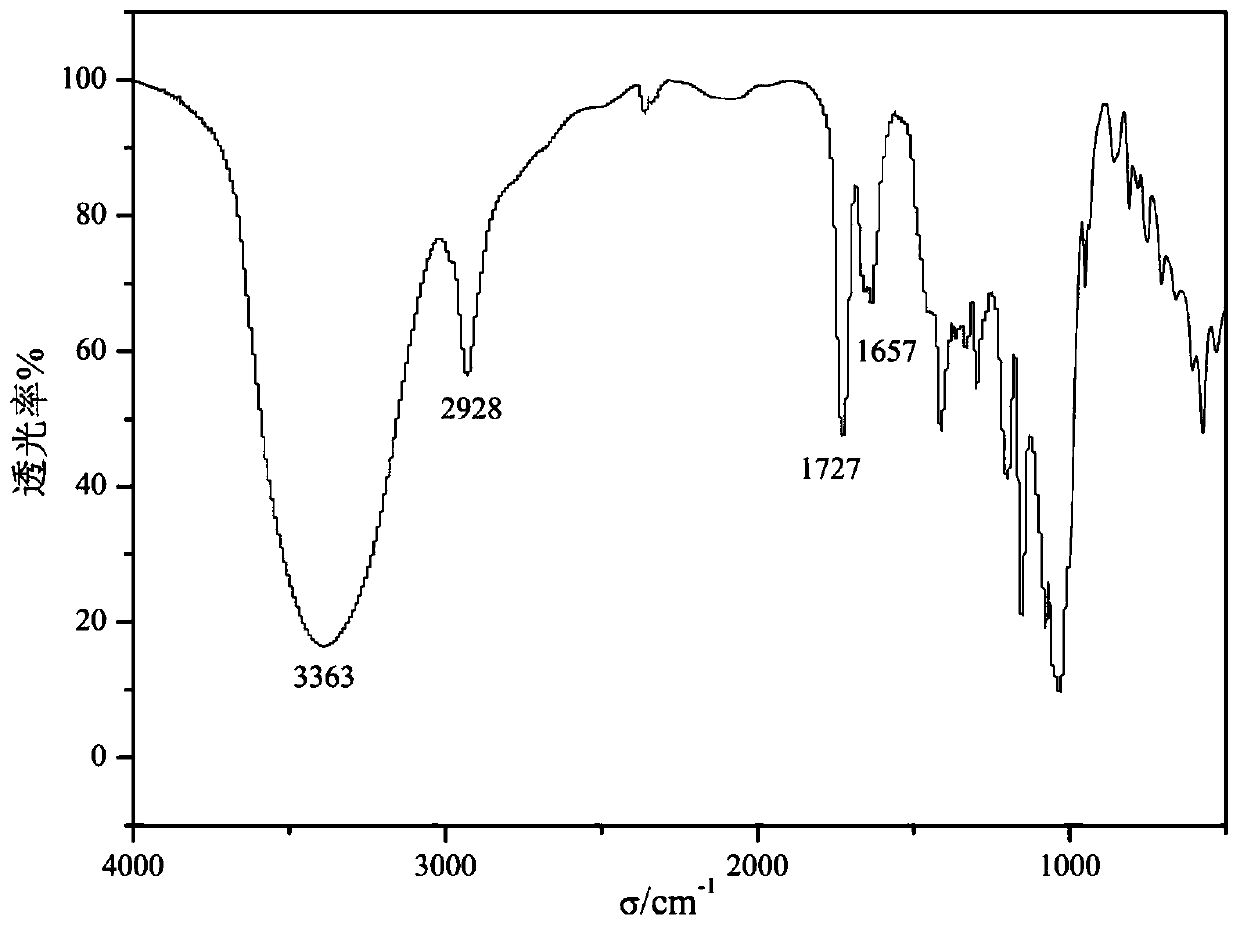
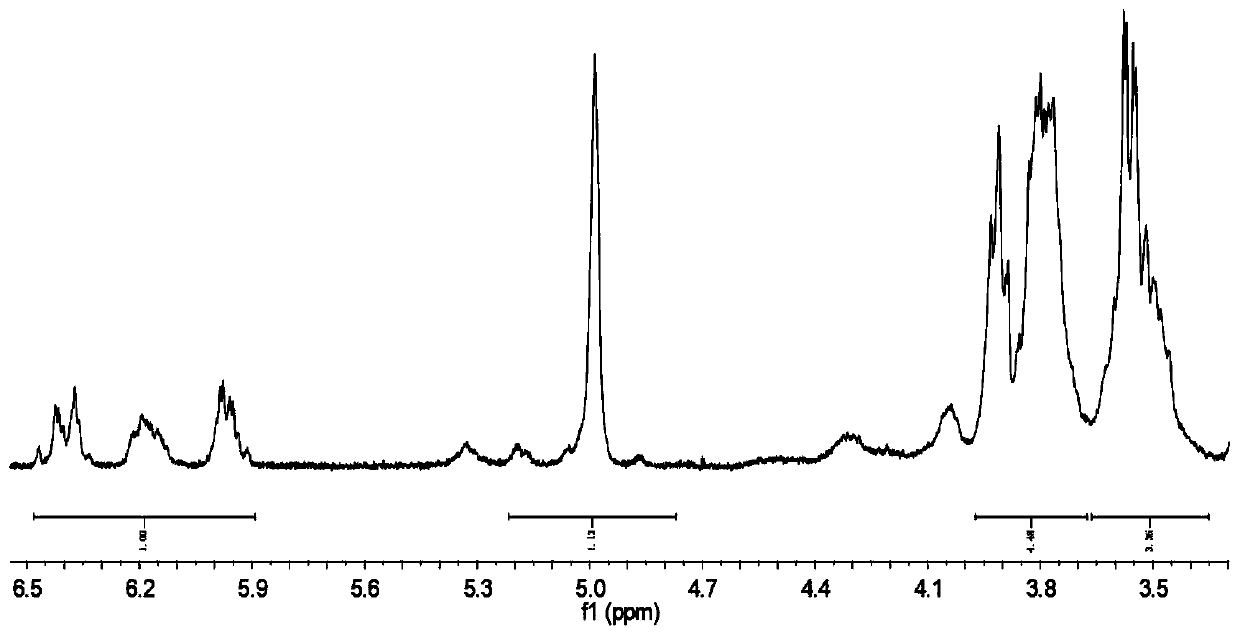
[0033] (1) Preparation of α-cyclodextrin ester;
[0034] Weigh 1.94g of α-cyclodextrin and dissolve it in 20mL of dimethylformamide, stir it magnetically to dissolve it completely, then add 3mL of triethylamine dropwise, and place it in an ice-water bath at 0°C for 5min with magnetic stirring. Under the condition of nitrogen protection, use a constant pressure dropping funnel to slowly add 2mL of acryloyl chloride dropwise to the flask, and react at room temperature for 30min after the dropwise addition is completed. Suction filtration again after precipitation, the obtained white solid was washed with acetone several times and suction filtration, the final product was placed in a vacuum oven, and dried at 70°C to constant weight to obtain α-cyclodextrin ester;
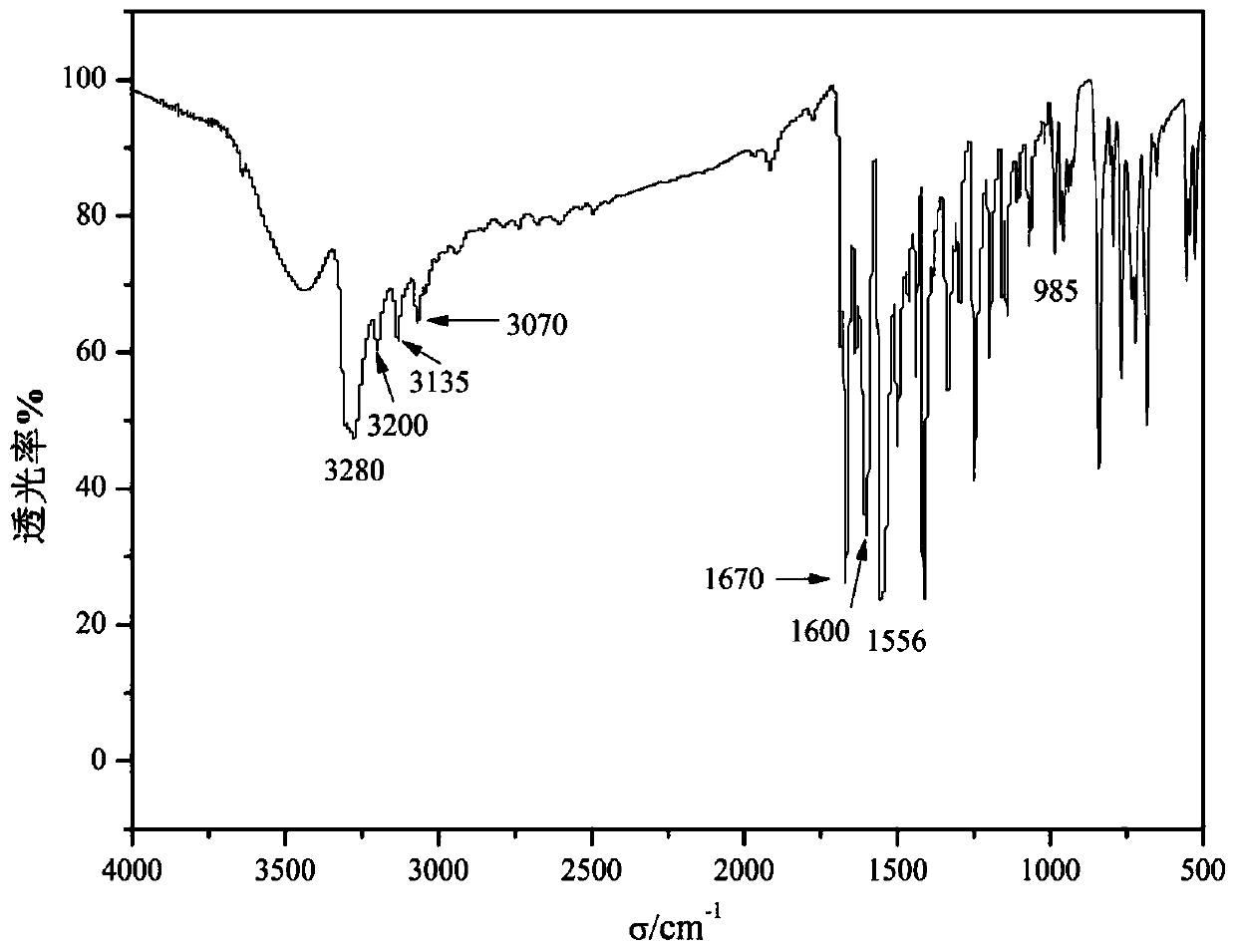
[0035](2) Preparation of acrylamido azobenzene;
[0036] Weigh 1.97g of p-aminoazobenzene and dissolve it in 20mL of benzene. After the magnetic stirring is completely dissolved, add 1.5mL of triethylamine. At room t...
Embodiment 2
[0039] (1) Preparation of α-cyclodextrin ester;
[0040] Weigh 1.94g of α-cyclodextrin and dissolve it in 15mL of dimethylformamide, stir it magnetically to dissolve it completely, then add 2.5mL triethylamine dropwise, and place it in an ice-water bath at 0°C for 3min with magnetic stirring , under the condition of nitrogen protection, slowly drop 1.5mL of acryloyl chloride into the flask with a constant pressure dropping funnel, and react at room temperature for 20min after the addition is completed. Precipitate with a large amount of acetone and then suction filter again. The obtained white solid is washed with acetone several times and suction filtered. The final product is placed in a vacuum oven and dried at 60°C to constant weight to obtain α-cyclodextrin ester;
[0041] (2) Preparation of acrylamido azobenzene;
[0042] Weigh 1.97g of p-aminoazobenzene and dissolve it in 15mL of benzene. After the magnetic stirring is completely dissolved, add 2.5mL of triethylamine. ...
Embodiment 3
[0045] (1) Preparation of α-cyclodextrin ester;
[0046] Weigh 1.94g of α-cyclodextrin and dissolve it in 18mL of dimethylformamide, stir it magnetically to dissolve it completely, then add 2mL of triethylamine dropwise, and place it in an ice-water bath at 0°C for 3min with magnetic stirring. Under the condition of nitrogen protection, use a constant pressure dropping funnel to slowly add 1mL of acryloyl chloride dropwise to the flask. After the dropwise addition, react at room temperature for 40min. After the reaction, vacuum filter to remove the precipitated solid. Suction filtration again after precipitation, the obtained white solid was washed with acetone several times and suction filtration, the final product was placed in a vacuum oven, and dried at 80°C to constant weight to obtain α-cyclodextrin ester;
[0047] (2) Preparation of acrylamido azobenzene;
[0048] Weigh 1.97g of p-aminoazobenzene and dissolve it in 10mL of benzene. After the magnetic stirring is comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com