Method for preparing large-granule industrial-grade urea phosphate product through continuous reaction crystallization of wet-process phosphoric acid
A wet-process phosphoric acid and reactive crystallization technology, applied in the preparation of urea derivatives, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of limited production and application, low purity of urea phosphate, low impurity removal rate, etc. Overcome the effects of high raw material cost, low impurity content and large particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
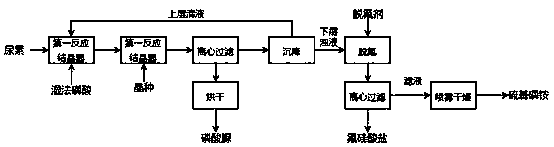
[0030] Wet-process phosphoric acid (45%, P 2 o 5 , F content 1.0%, SO 4 2- 4.2%) at 6.4 kg / h, urea at 2.35 kg / h, and circulating mother liquid at 12.8 kg / h are continuously sent to the first reaction crystallizer, the residence time of the feed liquid is 45 minutes, and the temperature of the reactor is controlled at 45°C to meet The feed liquid of the reaction time is sent to the second crystallizer continuously, and the temperature of the second crystallizer is controlled at 20 ℃, with 10g / h continuously adding the urea phosphate crystal seed that the average particle diameter is 117 microns, and the feed liquid residence time is 90 minutes, The stirring speed is 150 r / min, and the crystal slurry with a solid content of about 30% is continuously sent to the centrifuge, and separated by centrifugal filtration. The solid is the first filtered solid. After drying, 4.8kg of urea phosphate solid is obtained per hour. The centrifuged mother liquor is the first filtrate sent to...
Embodiment 2
[0033] With reference to the above-mentioned embodiment scheme, concentration is 35% wet-process phosphoric acid (35%, P 2 o 5 , F content 0.7%, SO 4 2- 3.3%) at 6.4 kg / h, urea at 1.8 kg / h, and circulating mother liquid at 9.6 kg / h are continuously sent to the first reaction crystallizer, the residence time of the feed liquid is 15 minutes, and the temperature of the reactor is controlled at 90°C to meet The feed liquid of the reaction time is sent to the second crystallizer continuously, and the temperature of the second crystallizer is controlled at 30 ℃, with 5g / h continuously adding the urea phosphate crystal seed that the average particle diameter is 70 microns, and the feed liquid residence time is 120 minutes, The stirring speed is 150 r / min, and the crystal slurry with a solid content of about 30% is continuously sent to the centrifuge for centrifugal filtration and separation. The solid is the first filtered solid. After drying, 3.8kg of urea phosphate solid is obt...
Embodiment 3
[0036] With reference to the above-mentioned embodiment scheme, the concentration is 75% wet-process phosphoric acid (75%, P 2 o 5 , F content 2.0%, SO 4 2- 8%) at 5.0kg / h, urea at 3.2 kg / h, and circulating mother liquor at 20 kg / h are continuously sent to the first reaction crystallizer, the residence time of the feed liquid is 60 minutes, and the temperature of the reactor is controlled at 40°C, satisfying The feed liquid of the reaction time is sent to the second crystallizer continuously, and the temperature of the second crystallizer is controlled at 10 ℃, with 25g / h continuously adding the urea phosphate crystal seed that the average particle diameter is 150 microns, and the feed liquid residence time is 30 minutes, The stirring speed is 150 r / min, and the crystal slurry with a solid content of about 30% is continuously sent to the centrifuge, and separated by centrifugal filtration. The solid is the first filtered solid. After drying, 7.2kg of urea phosphate solid is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com