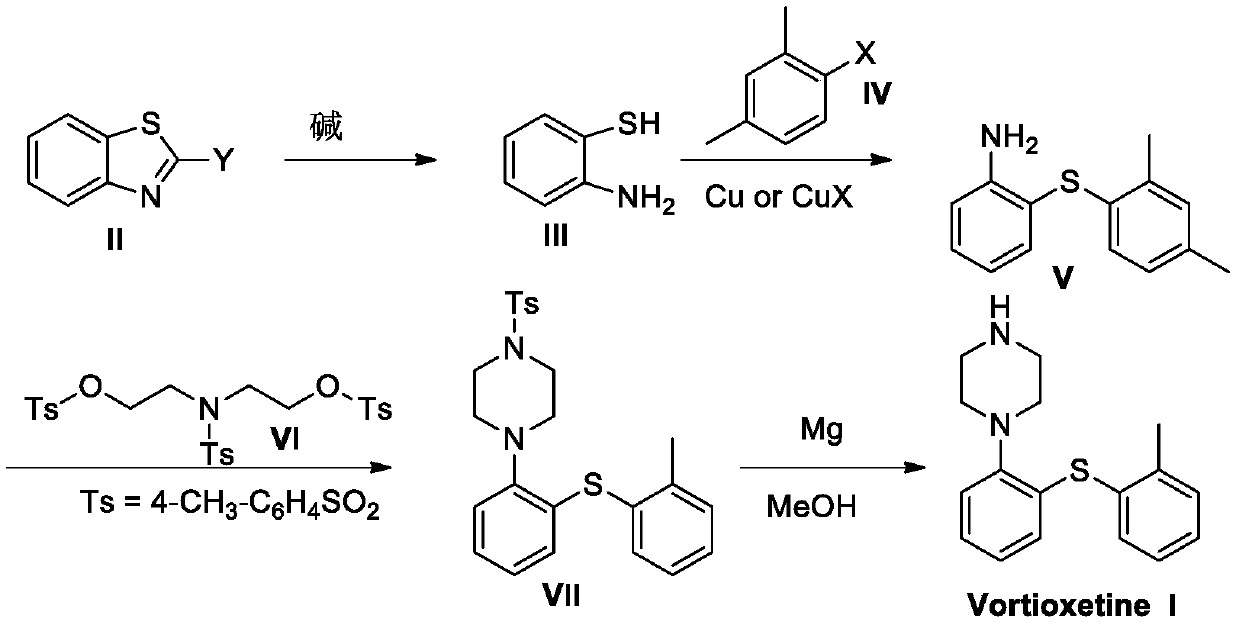
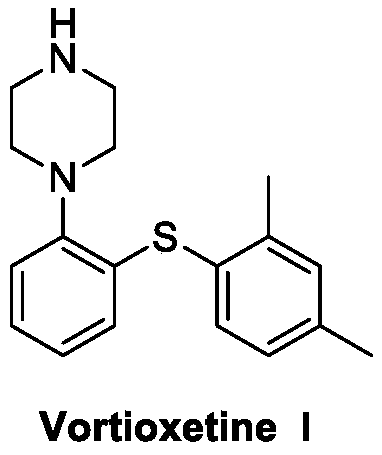
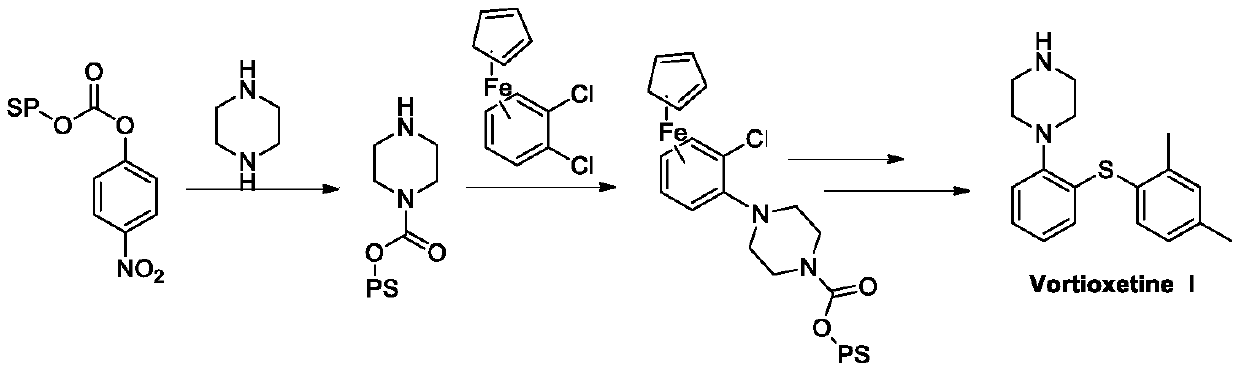
Preparation method of vortioxetine
A technology of vortioxetine and compounds, which is applied in the field of preparation of vortioxetine, can solve the problems of high price and unfavorable industrial production, and achieve the effects of low cost, easy availability of raw materials, and high purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of o-aminothiophenol (III):
[0057]
[0058] Weigh 41.9g (0.31mol, 1.0eq.) of benzothiazole and place it in a 2.0L reactor, add 85.6g (0.62mol, 2.0eq.) of potassium carbonate, 500mL PEG 200 into the reactor, and raise the temperature to 110 °C and stirred for 24 hours, and TLC detected that the reaction was complete. Add 500mL of water to the reaction solution, then add 500mL of ethyl acetate for extraction, distill under reduced pressure at 40°C to obtain a crude product, and then obtain 30.1g of a pure oily product by column chromatography. Yield 80%. 1 H-NMR (400MHz, CDCl 3 ):d 7.40(d,J=7.5Hz,1H),7.14(t,J=9.3Hz,1H),6.77-6.70(m,2H),4.08(br-s,2H),3.10(s,1H ).
Embodiment 2
[0060] The preparation of o-aminothiophenol (III):
[0061]
[0062] Weigh 41.5g (0.31mol, 1.0eq.) of benzothiazole and place it in a 2.0L reactor, add 34.7g (0.62mol, 2.0eq.) of potassium hydroxide and 500mL PEG 200 into the reactor, and heat up to Stir at 110° C. for 24 hours, and TLC detects that the reaction is complete. 500 mL of water was added to the reaction solution, and then 500 mL of ethyl acetate was added for extraction. The crude product was obtained by distillation under reduced pressure at 40° C., and then 31.8 g of pure oily product was obtained by column chromatography. Yield 85%. 1 H-NMR (400MHz, CDCl3): d 7.40 (d, J = 7.5Hz, 1H), 7.14 (t, J = 9.3Hz, 1H), 6.77-6.70 (m, 2H), 4.08 (br-s, 2H ), 3.10(s,1H).
[0063] For a more concise description, Embodiment 3 to Embodiment 12 are provided in the form of a table, and the specific operation steps of Embodiment 3 to Embodiment 12 refer to Embodiment 1, and the general reaction formula is as follows:
[0064...
Embodiment 13
[0068] 2-(2,4-Dimethylphenylthio)aniline preparation:
[0069]
[0070] Weigh 41.9g (0.31mol, 1.0eq.) of benzothiazole into a 2.0L reactor, add 85.6g of potassium carbonate (0.62mol, 2.0eq), and 500mL of PEG 200 into the reactor, and heat up to 110°C Stir for 24 hours, and TLC detects that the reaction is complete. Add 72.2g (0.39mol, 1.3eq.) 2,4-dimethylbromobenzene and 2.0g (31.00mmol, 10mol%) copper powder to a 2.0L reaction flask, and stir the reaction at 200°C for 22h under nitrogen protection. TLC showed that the reaction was relatively complete.
[0071] Post-reaction treatment: cool down to 25°C, add 300mL ethyl acetate and 400mL water, stir well for 15min to make it uniform, then filter with diatomaceous earth, wash the filter cake twice with 200mL ethyl acetate, separate the liquid, and use acetic acid for the water phase Ethyl ester (200mL×2) was back-extracted twice, the organic phase was combined, and the organic layer was washed once with water, then 15mL co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com