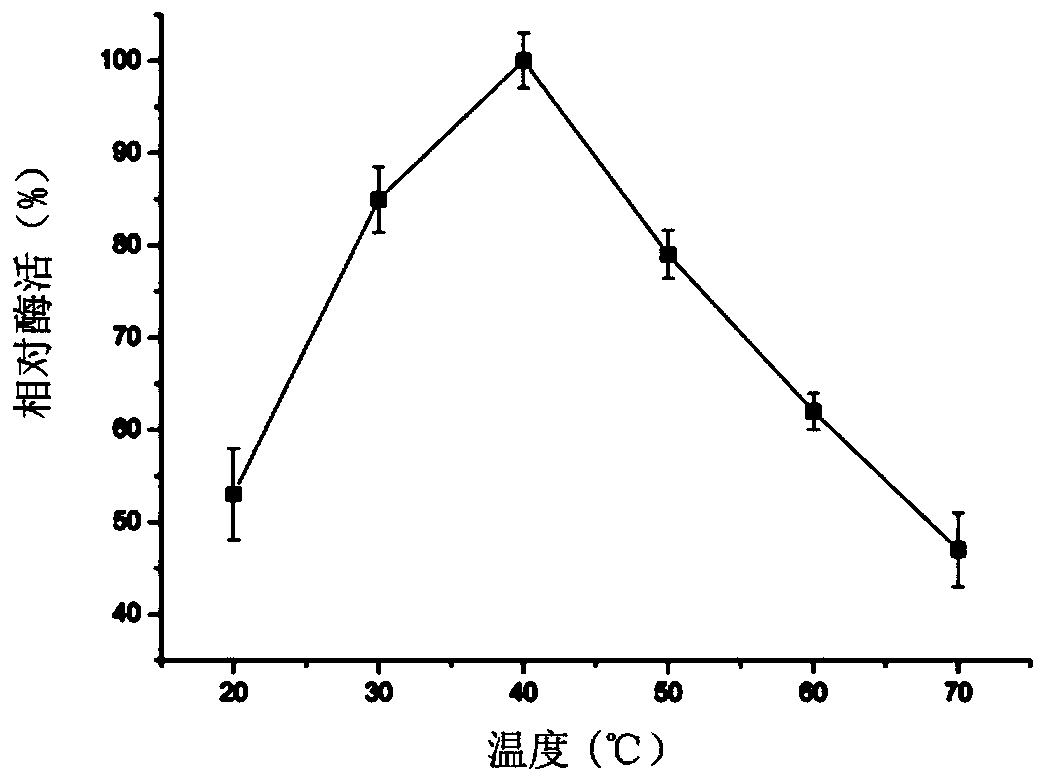
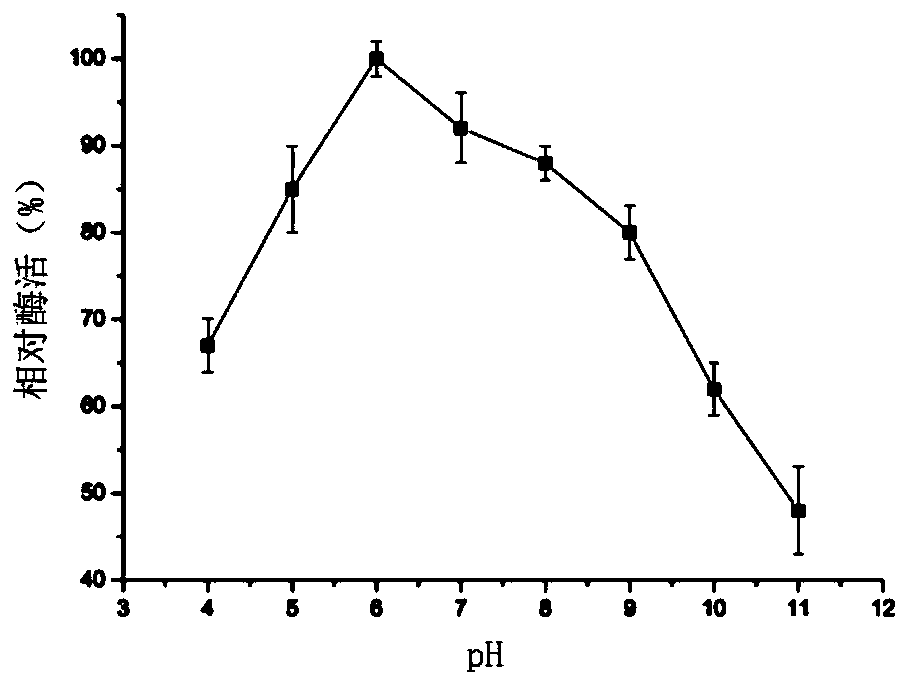
Lysozyme derived from phases and gene and application of lysozyme
A technology of lysozyme and gene, which is applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of narrow action temperature range, narrow action pH range, and few species, and achieve wide action temperature range, excellent genetic resources and enzymes resources, the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The acquisition of embodiment 1 lysK1 gene
[0027] The phage used in this example was isolated from seawater at a depth of 2800 m in the western Pacific Ocean, and the phage was long-tailed phage WP3 (phage WP3). Primer F (5'-ATCGGATCCATGAGCCCGACTGTAAGCGCCA-3') and primer R (5'-GGACTCGAGTCAGATCTTCGGTCCCTCCAGCCT-3') were used to PCR amplify the lysK1 gene fragment of the deep-sea phage, and BamH1 and Xho1 restriction sites were introduced into its upstream and downstream, respectively. The full-length CDS (Coding sequence, coding sequence) of the lysK1 gene is 1221 bp, and its nucleotide sequence is shown in SEQ ID NO: 2, encoding 406 amino acids.
Embodiment 2
[0028] Cloning expression of embodiment 2lysK1 gene
[0029] The lysK1 gene fragment obtained in Example 1 was connected between the BamH1 and Xho1 restriction sites of the pET28a vector to obtain the pET28a-lysK1 recombinant vector; the obtained pET28a-lysK1 recombinant vector was transformed into Escherichia coli E.coliBL21 (DE3) competent State cells, and smeared on LB solid culture plate containing 10mg / ml kanamycin and 10% agar, cultured at 37°C for 24 hours; In LB liquid medium, culture overnight at 37°C with shaking at 200rpm; inoculate the positive clones cultured overnight at a dilution ratio of 1:100 into 500ml of LB liquid medium (containing 10mg / ml kanamycin), at 37°C , shake culture at 200rpm to OD 600 When it reaches 0.6, add isopropylthio-β-D-galactoside (IPTG) at a final concentration of 1 mM, and induce the expression of LysK1 lysozyme at 18° C. for 12 hours.
Embodiment 3
[0030] The separation and purification of embodiment 3LysK1 protein
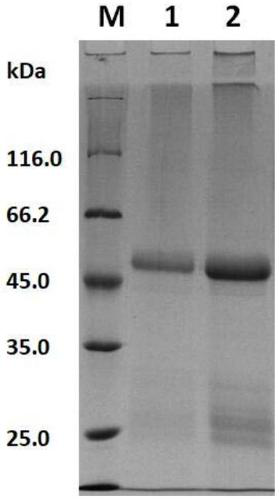
[0031] 15000rpm centrifugation collects the thalli that embodiment 2 induces to express, after washing three times with PBS, with lysis buffer (0.3mol / L NaCl, 10mmol / L imidazole, 50mmol / L NaH 2 PO 4 , pH 8.0) resuspended cells. Use the sonication method (power 80%, 5s / 5s pulse cycle) to break and lyse the bacteria on ice, centrifuge at 15000rpm at a high speed, and collect the supernatant; rotate and mix the collected supernatant with Ni-NTA Agarose for 30min According to the instructions of the Ni-NTA Agarose kit (QIAGEN), the lysK1 lysozyme was purified to obtain the LysK1 protein, whose amino acid sequence is shown in SEQ ID NO: 1, and analyzed by SDS-PAGE electrophoresis. figure 1 , Lanes 1 and 2 are the purified recombinant LysK1 protein, and its size is consistent with the predicted molecular weight (48.5kD).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com