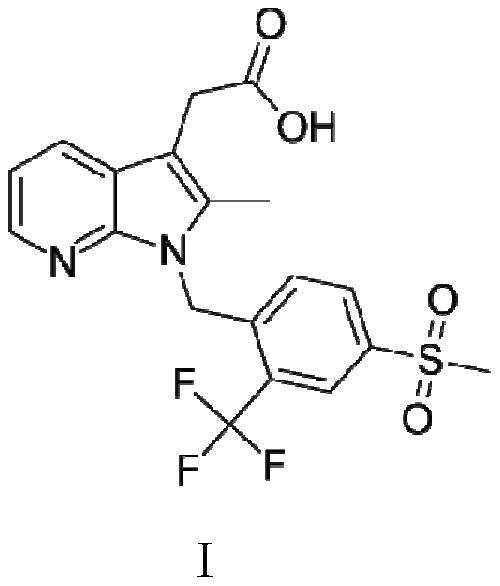
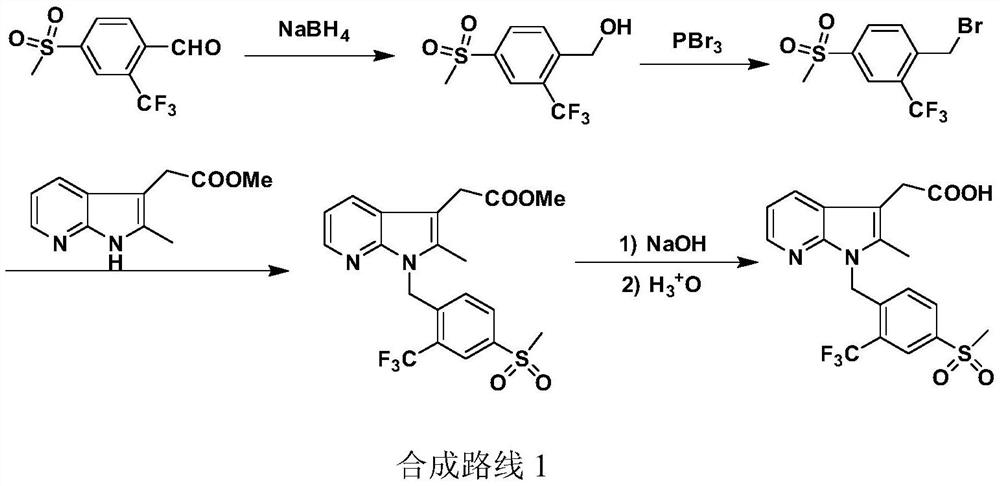
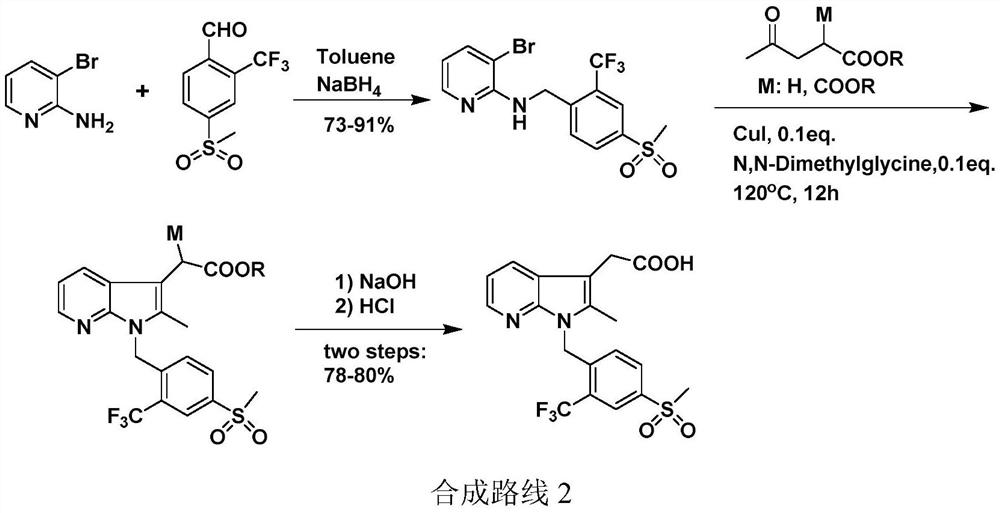
A kind of convenient preparation method of prostaglandin D2 receptor inhibitor compound
A technology of receptor inhibitors and prostaglandins, applied in the field of medicinal chemistry, can solve the problems of high price of 2-amino-3-bromopyridine, unfavorable green industrial production, and large amount of metal residues, so as to achieve low production of three wastes and low cost Low, high yield and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of Fevipiprant (Ⅰ)
[0055] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, water trap and reflux condenser, add 200 grams of toluene, 25.3 grams (0.1 mole) 4-methanesulfonyl-2-trifluoromethylbenzylamine (Ⅱ) , 21.0 g (0.1 mol) dimethyl 3-acetylglutarate (III 1 ), 0.5 g of p-toluenesulfonic acid, stirred and reacted at 45 to 50° C. for 2 hours, and stirred and reacted at 82 to 85° C. for 4 hours, while separating the water separated by azeotropy. Cool to 20-25° C., add 1.0 g DBU, 10.0 g (0.11 mole) 2-chloroacrolein, and stir at 40-45° C. for 4 hours. Add 40.0 g (0.4 mol) of 17 wt% ammonia methanol solution, and stir the reaction at 50 to 55°C for 4 hours. Cool to 20 to 25°C, add 250 grams of water, 30 grams of 20wt% sodium hydroxide aqueous solution, stir and react at 25 to 30°C for 2 hours, separate layers, add 0.5 grams of activated carbon to the separated water layer, and set the temperature at 25 t...
Embodiment 2
[0059] Embodiment 2: the preparation of Fevipiprant (Ⅰ)
[0060] To a 500 ml four-neck flask connected with stirring, thermometer, water separator and reflux condenser, add 200 g of 1,2-dichloroethane, 25.3 g (0.1 mole) of 4-methylsulfonyl-2-trifluoro Methylbenzylamine (II), 23.0 g (0.1 mol) diethyl 3-acetylglutarate (III 2 ), 0.5 gram of 98wt% concentrated sulfuric acid, stirred and reacted at 45 to 50° C. for 2 hours, and stirred and reacted at 70 to 75° C. for 5 hours, while separating the water separated by azeotropy. Cool to 20 to 25°C, add 1.2 g of DBU, 13.5 g (0.1 mole) of 2-bromoacrolein, and react with stirring at 35 to 40°C for 4 hours. Add 40.0 g (0.4 mol) of 17 wt% ammonia methanol solution, and stir the reaction at 50 to 55°C for 4 hours. Cool to 20 to 25°C, add 250 grams of water, 30 grams of 20wt% sodium hydroxide aqueous solution, stir and react at 20 to 25°C for 2 hours, separate layers, add 0.5 grams of activated carbon to the separated water layer, and set...
Embodiment 3
[0061] Embodiment 3: the preparation of Fevipiprant (Ⅰ)
[0062] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, water trap and reflux condenser, add 200 grams of toluene, 25.3 grams (0.1 mole) 4-methanesulfonyl-2-trifluoromethylbenzylamine (Ⅱ) , 21.0 g (0.1 mol) dimethyl 3-acetylglutarate (III 1 ), 0.5 g of p-toluenesulfonic acid, stirred and reacted at 40 to 45° C. for 3 hours, and stirred and reacted at 82 to 85° C. for 4 hours, while separating the water separated by azeotropy. Cool to 20-25°C, add 1.2g of DBU, 13.5g (0.1 mole) of 2-bromoacrolein, and react with stirring at 40-45°C for 4 hours. Add 40.0 g (0.4 mol) of 17 wt% ammonia methanol solution, and stir the reaction at 50 to 55°C for 4 hours. Cool to 20 to 25°C, add 250 grams of water, 35 grams of 20wt% potassium hydroxide aqueous solution, stir and react at 20 to 25°C for 2 hours, separate layers, add 0.5 grams of activated carbon to the separated water layer, and set the tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com