Controllable preparation method and application of supported non-noble metal diatomic catalyst
A non-precious metal, diatomic technology, applied in the field of catalysis, can solve the problems of high cost of precious metal elements, restricting the scale and scope of application, and achieve the effect of simple and feasible operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
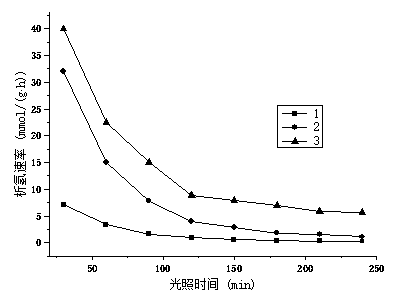
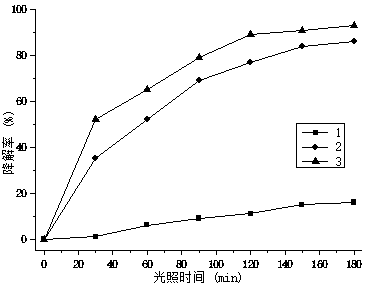
Image
Examples
Embodiment 1
[0020] (1) After heating 20mL of deionized water to 40°C, add 0.36g of boric acid, 0.32g of sodium citrate, 0.02g of glycine, 0.26g of nickel sulfate, and 0.23g of cobalt sulfate, and stir thoroughly. After the solid matter is completely dissolved, Stop heating, cool to room temperature naturally, make solution A;
[0021] (2) The pH value of solution A is adjusted to 12 by sodium hydroxide solution to prepare solution B;
[0022] (3) After heating solution B to 60°C, add 0.35 g of g-C to solution B 3 N 4 powder, after stirring at 60°C for 30 minutes, solution C was prepared;
[0023] (4) Add 0.56g of sodium hypophosphite into 20mL of deionized water, and stir to prepare solution D after the solid matter is completely dissolved;
[0024] (5) Pour solution D into solution C, react at 60°C for 50 minutes, filter and put the solid matter in an oven at 50°C for 4 hours to obtain g-C 3 N 4 Supported NiCo two-atom catalysts.
Embodiment 2
[0026] (1) After heating 100mL of deionized water to 50°C, add 1.25g of boric acid, 1.5g of sodium citrate, 0.11g of glycine, 1.58g of nickel chloride, and 1.2g of cobalt nitrate, and stir thoroughly until the solid matter is completely dissolved , stop heating, and naturally cool to room temperature to prepare solution A;
[0027] (2) The pH value of solution A is adjusted to 11 by sodium hydroxide solution to prepare solution B;
[0028] (3) After heating solution B to 50°C, add 1.8 g of g-C to solution B 3 N 4 powder, after stirring at 50°C for 40 minutes, solution C was prepared;
[0029] (4) 1.5g of sodium hypophosphite and 1.5g of potassium hypophosphite were added to 100mL of deionized water, and stirred to prepare solution D after the solid matter was completely dissolved;
[0030] (5) Pour solution D into solution C, react at 50°C for 1.5h, filter and put the solid matter in an oven at 50°C for 6h to obtain g-C 3 N 4 Supported NiCo two-atom catalysts.
Embodiment 3
[0032] (1) After heating 150mL of deionized water to 60°C, add 1.75g of boric acid, 2.2g of sodium citrate, 0.18g of glycine, 1.89g of nickel nitrate, and 2.2g of cobalt chloride, and stir thoroughly until the solid matter is completely dissolved , stop heating, and naturally cool to room temperature to prepare solution A;
[0033] (2) The pH value of solution A is adjusted to 10 by sodium hydroxide solution to prepare solution B;
[0034] (3) After heating solution B to 65°C, add 2.2 g of g-C to solution B 3 N 4 powder, after stirring at 65°C for 50 minutes, solution C was prepared;
[0035] (4) Add 3.8g of sodium hypophosphite into 50mL of deionized water, stir until the solid matter is completely dissolved to prepare solution D;
[0036] (5) Pour solution D into solution C, react at 65°C for 45 minutes, filter and put the solid matter in an oven at 50°C for 8 hours to obtain g-C 3 N 4 Supported NiCo two-atom catalysts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com