Preparation method and application of high-purity dimethyl 3-isobutylglutarate
A technology of isobutylglutaric acid dimethyl ester and isobutyl glutaric acid, applied in the preparation of high-purity 3-isobutylglutaric acid dimethyl ester, high-purity 3-isobutylglutaric acid dimethyl The application field of methyl ester in the further preparation of pregabalin can solve the problems of many impurities, low purity of dimethyl 3-isobutylglutarate, and removal of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The preparation method of pregabalin
[0085] One object of the present invention is to provide a method for preparing high-purity pregabalin.
[0086] In a specific embodiment, the invention provides a kind of preparation method of pregabalin, it comprises steps:
[0087] (1) In methanol, in the presence of potassium permanganate and concentrated sulfuric acid, 3-isobutylglutaric acid (formula 1) is reacted to obtain 3-isobutylglutaric acid dimethyl ester (formula 2),
[0088] with
[0089] (2) Pregabalin was prepared from the dimethyl 3-isobutylglutarate (formula 2) obtained in step (1).
[0090] In another preference, step (2) includes the steps of:
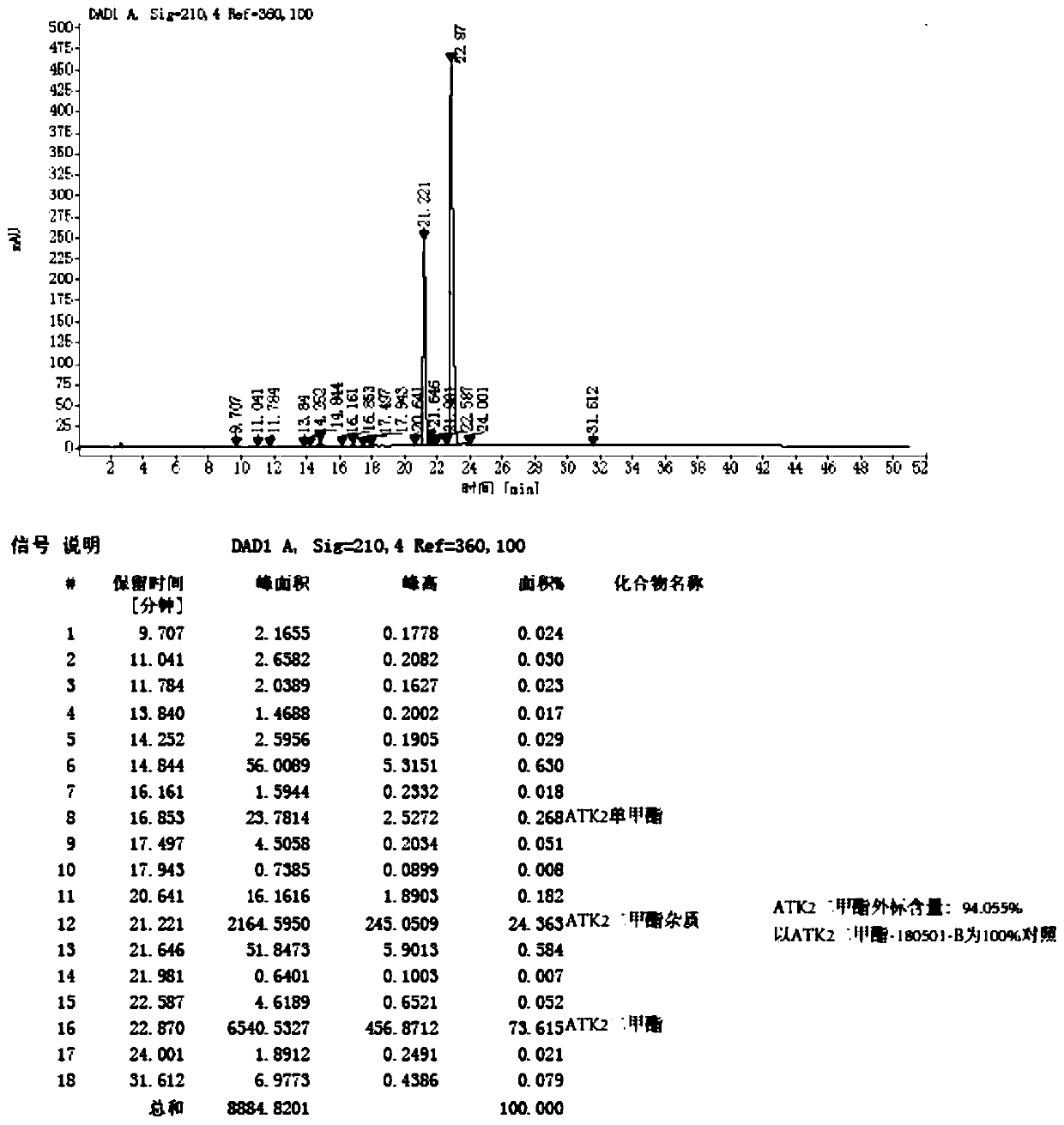
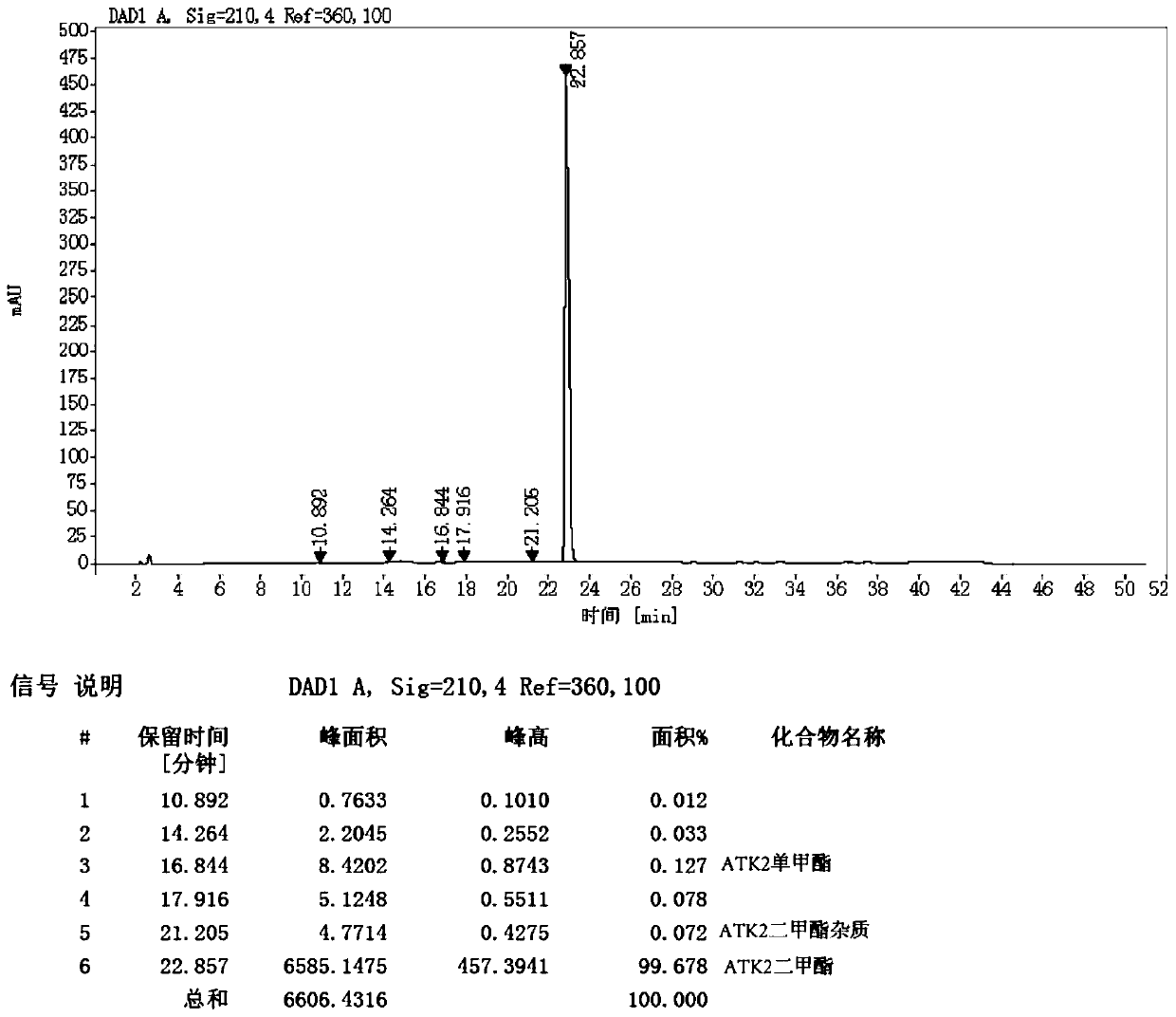
[0091] 3-isobutylglutaric acid dimethyl ester (formula 2) obtains the formula 9 compound through enzymatic selective hydrolysis; The formula 9 compound obtains the formula 10 compound with ammoniacal liquor ammonia solution; And the formula 10 compound carries out Hoffman degradation with sodium hypochlorite to ob...
Embodiment 2
[0133] Example 2: Preparation of 2-cyano-5-methyl-2-enhexanoic acid methyl ester (formula 6) (preparation of impurity 6 reference substance)
[0134] Add isovaleraldehyde (43g, 0.5mol), cyanoacetamide (84g, 1mol) and water (500ml) into the reaction flask at one time, slowly add piperazine (15g, 0.17mol) at a low temperature of 10°C, keep warm after adding After the reaction, a white solid was gradually formed, and toluene (250ml) was added and stirred for 10-12h to obtain 2,6-dicyano-3-isobutylglutaramide (Formula 5). In order to obtain more impurity 6 as a reference substance, the compound of formula 5 was prepared in a way that is more likely to form the impurity of formula 7 in this example.
[0135] The 2,6-dicyano-3-isobutylglutaramide (formula 5) generated in the previous step reaction was added dropwise to concentrated sulfuric acid (300 g). After the dropwise addition, the temperature was slowly raised to reflux for 12 hours, cooled to 80°C, the organic phase was sepa...
Embodiment 3
[0140] Example three: Preparation of dimethyl 3-isobutylglutarate (formula 2) (research on the amount of potassium permanganate)
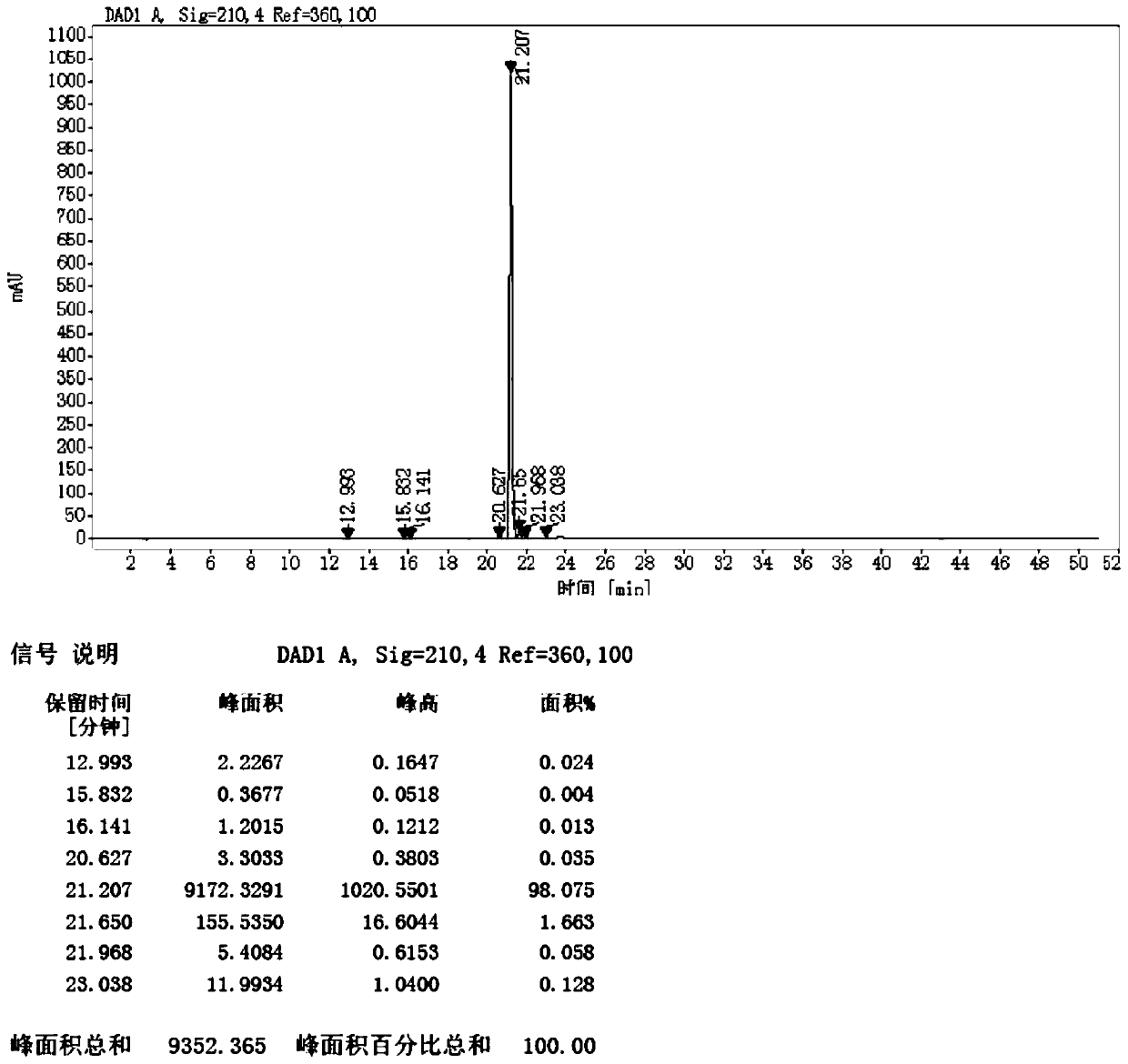
[0141] Get 6 reaction flasks of 500ml, add 18.8g of 3-isobutylglutaric acid (formula 1) respectively, then respectively add 188g of methanol, after stirring and dissolving, add potassium permanganate 0.94g, potassium permanganate 0.94g, 1.88g, 3.76g, 5.64g, 9.4g. After the addition, 1.88g of concentrated sulfuric acid was added dropwise to the 6 reaction flasks, and the temperature was raised to reflux for 10-12 hours. The reaction liquid was cooled to room temperature, then methanol was distilled off, the residual liquid was extracted with n-hexane, washed with sodium bicarbonate solution until neutral, the organic layer was dried, filtered, and concentrated to obtain the crude product of 3-isobutylglutaric acid dimethyl ester (Formula 2) Detect product and impurity formula 6 data with high performance liquid phase as shown in table 1:
[0142] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com