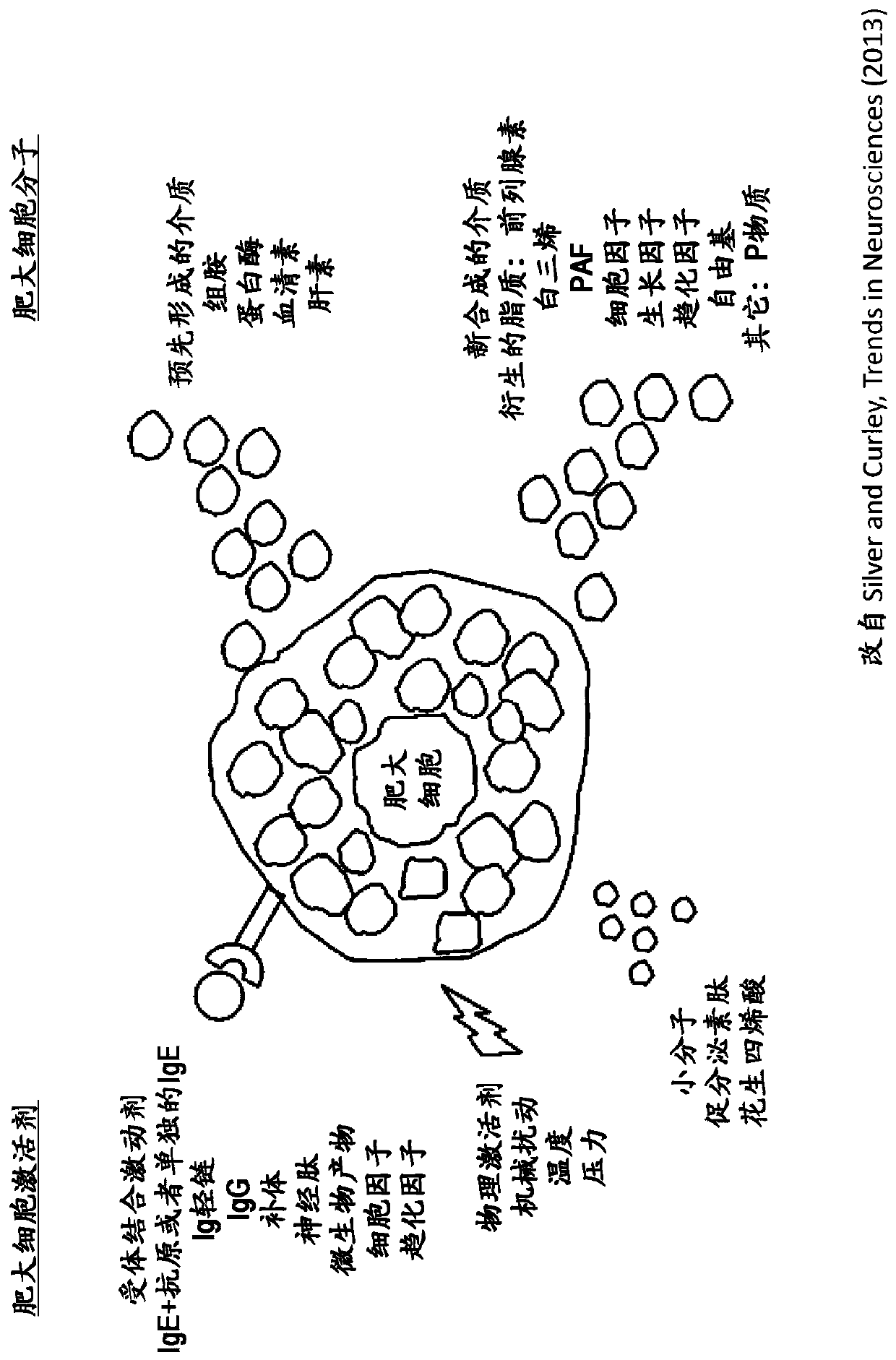
Cannabinoid-containing complex mixtures for the treatment of mast cell-associated or basophil-mediated inflammatory disorders
A cannabinoid, cannabis technology, applied in the treatment and prevention of chronic and acute inflammatory diseases in mammals, the field of pharmaceutical compositions containing this complex mixture, can address the safety, efficacy and consistency of plant-derived medicines that have not approached the To reduce the ethical burden and reduce the regulatory burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
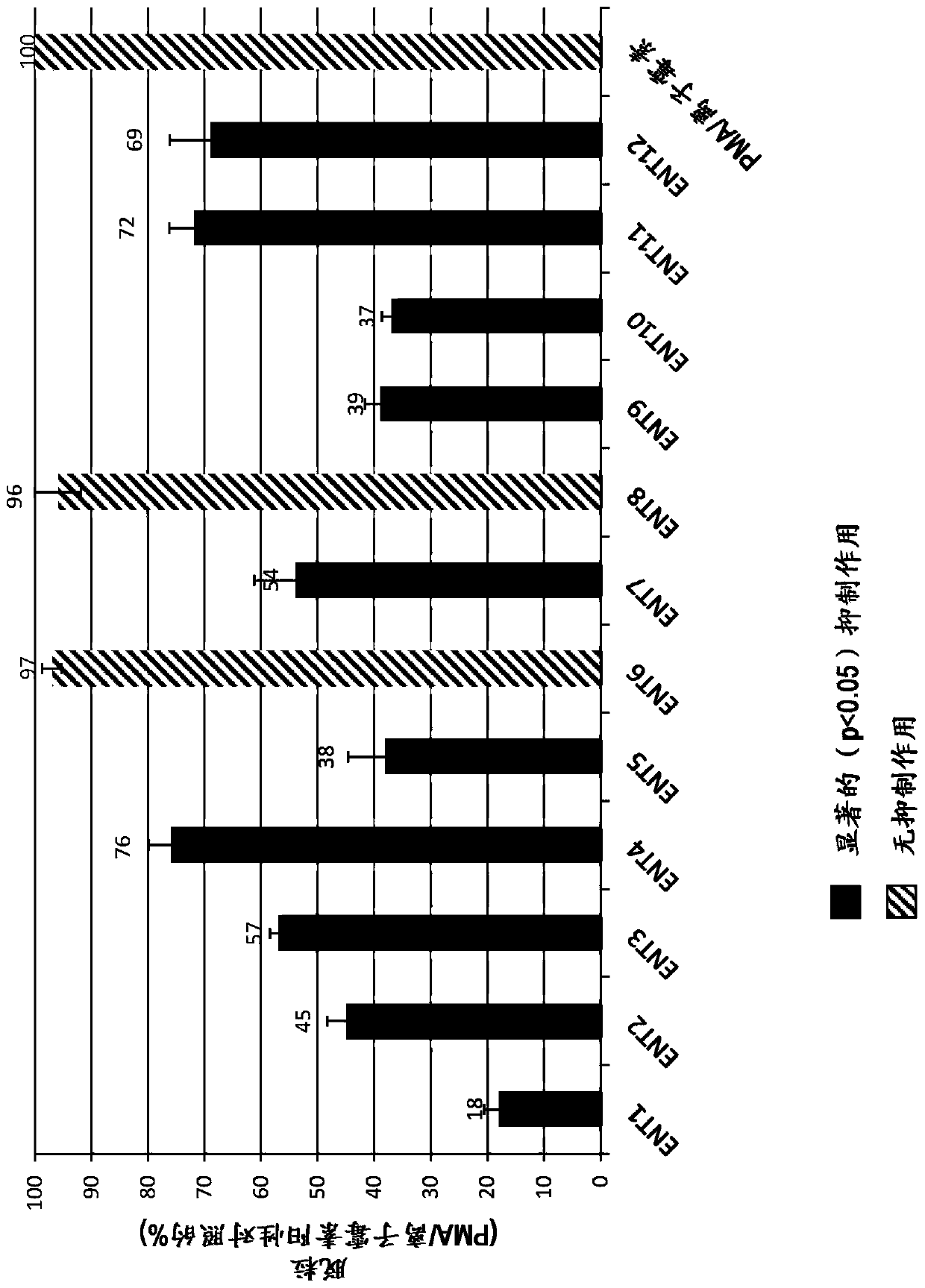
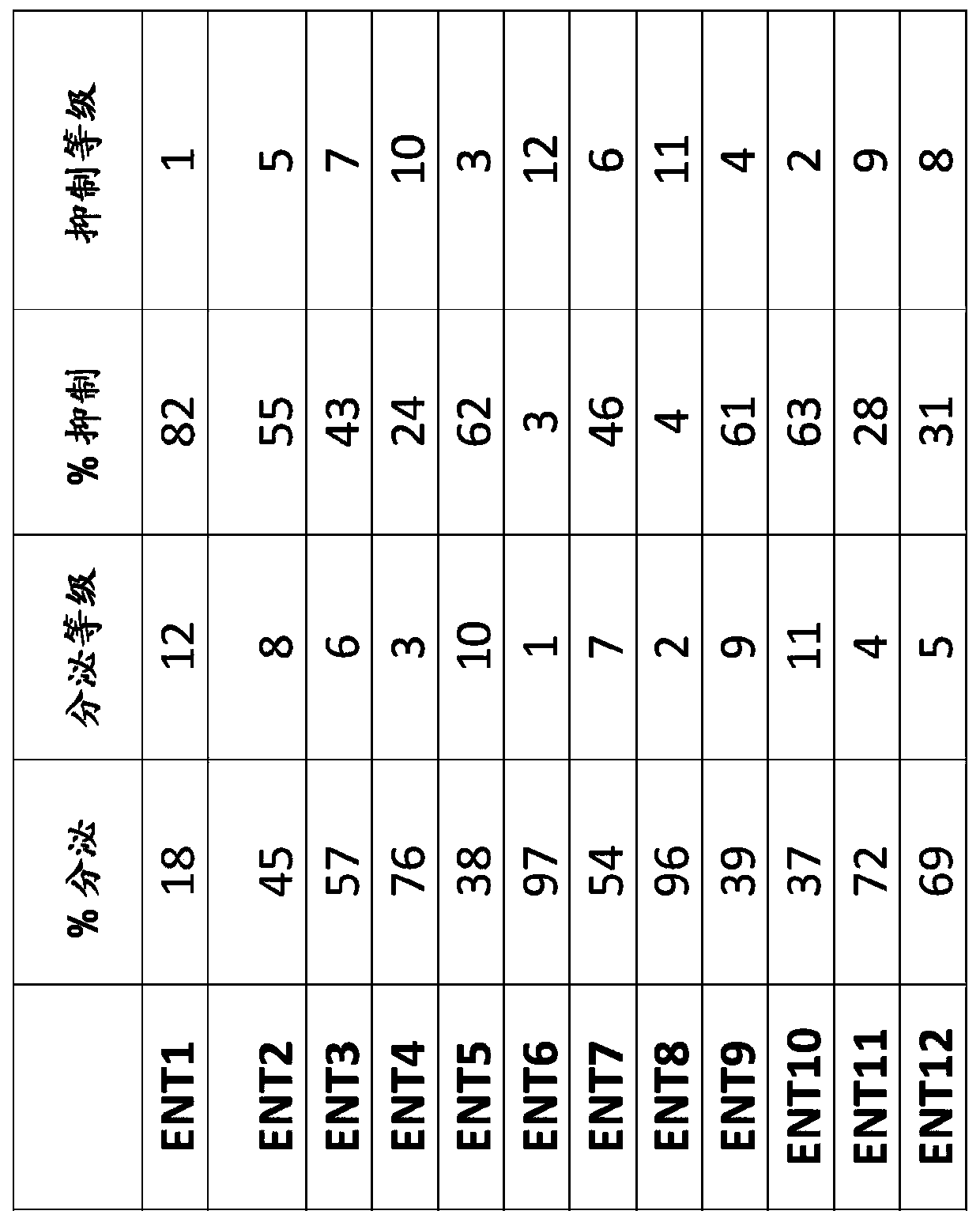
[0228] 5.9.1. Example 1: Submixtures Containing Minor Cannabinoids and / or Terpenes (ENT 1-ENT12A)
[0229]Twenty-one different subblends (ENT 1-ENT 12A) containing minor cannabinoids and / or terpenes were generated by mixing the individual components specified in Table 1. Components were obtained from various suppliers - nerolidol (#N0454) from Tokyo Chemical Industry, linalool (#L0048) from Tokyo Chemical Industry, a-pinene (#P45680) from Sigma Aldrich, from MP Limonene (#155234) from Biomedicals, Phytol (#FLMS-035) from Ultr Scientific, Cannabidiol (#C-140) from Sigma Aldrich, Cannabichromene (#C-143) from Sigma Aldrich , Cannabidiol (#C-045) from Sigma Aldrich, Cannabigerol (#C-141) from Sigma Aldrich and Cannabidiol (#C-046) from Sigma Aldrich. Each component is added in such an amount that each submixture contains the same molar concentration of each individual component.
[0230] Similarly, a cannabinoid-containing complex mixture was created by mixing the main cannabin...
Embodiment 2
[0231] 5.9.2. Example 2: Anti-inflammatory effect of cannabinoid-containing complex mixtures measured based on the inhibition of histamine release
[0232] The anti-inflammatory effects of various cannabinoid-containing complex mixtures comprising cannabidiol and one of twelve sub-blends (ENT1-12) were tested by an FcεRI linkage-based in vitro assay.
[0233] FcεRI is a high-affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in allergy and parasite immunity. FcεRI is multimeric and a member of a family of related antigen / Fc receptors that share conserved structural features and similar roles in initiating intracellular signaling cascades. In humans, FcεRI controls the activation of mast cells and basophils and is involved in IgE-mediated antigen presentation. Multivalent antigens bind through FcεRI and cross-link IgE molecules held on the cell surface. Receptor aggregation induces multiple signaling pathways that control different eff...
Embodiment 6
[0262] 5.9.6. Example 6: Cellular and molecular mechanisms of anti-inflammatory effects of complex mixtures containing cannabinoids
[0263] Cannabigerol (CBG) can have anti-inflammatory effects by (1) inhibiting secretory pathways or (2) stimulating antisecretory pathways or both, i.e. inhibiting degranulation. Activation of the Gi-coupled receptors CB1 and CB2 induces Gi-mediated inhibition of adenylyl cyclase and subsequent reduction in intracellular cAMP concentration. Since CBG is an antagonist against CB1 and CB2, both expressed on RBL mast cells, inhibition of CB1 and CB2 by CBG can increase intracellular cAMP and inhibit mast cell degranulation. Increased cAMP is known to inhibit degranulation of mast cells.
[0264] CB1 and CB2 receptors can be constitutively activated by their endogenous ligands such as anandamide (arachidonyl ethanolamine or AEA). AEA is metabolized by fatty acid amide hydrolase (FAAH), therefore, inhibition of FAAH can increase endogenous AEA lev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com