Nucleotide sequence of human inhibin A and recombinant expression method for human inhibin A
A nucleotide sequence and inhibin technology, applied in recombinant DNA technology, nucleic acid vectors, chemical instruments and methods, etc., can solve the problems of poor antibody specificity, gap between prokaryotic and eukaryotic expression systems, low activity of INHA protein, etc., to achieve Good stability and expression-promoting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Cloning and sequence analysis of human inhibin A
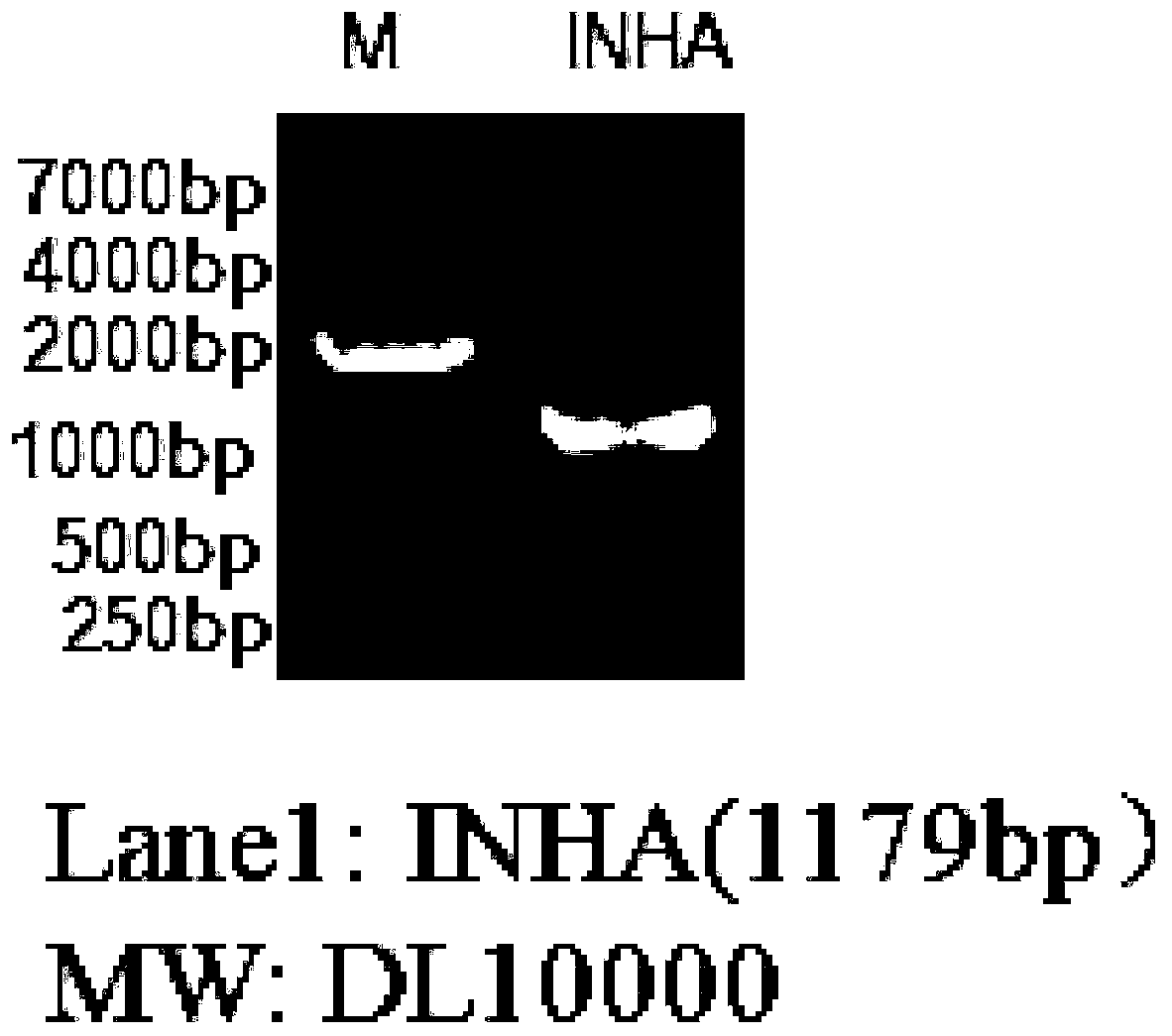
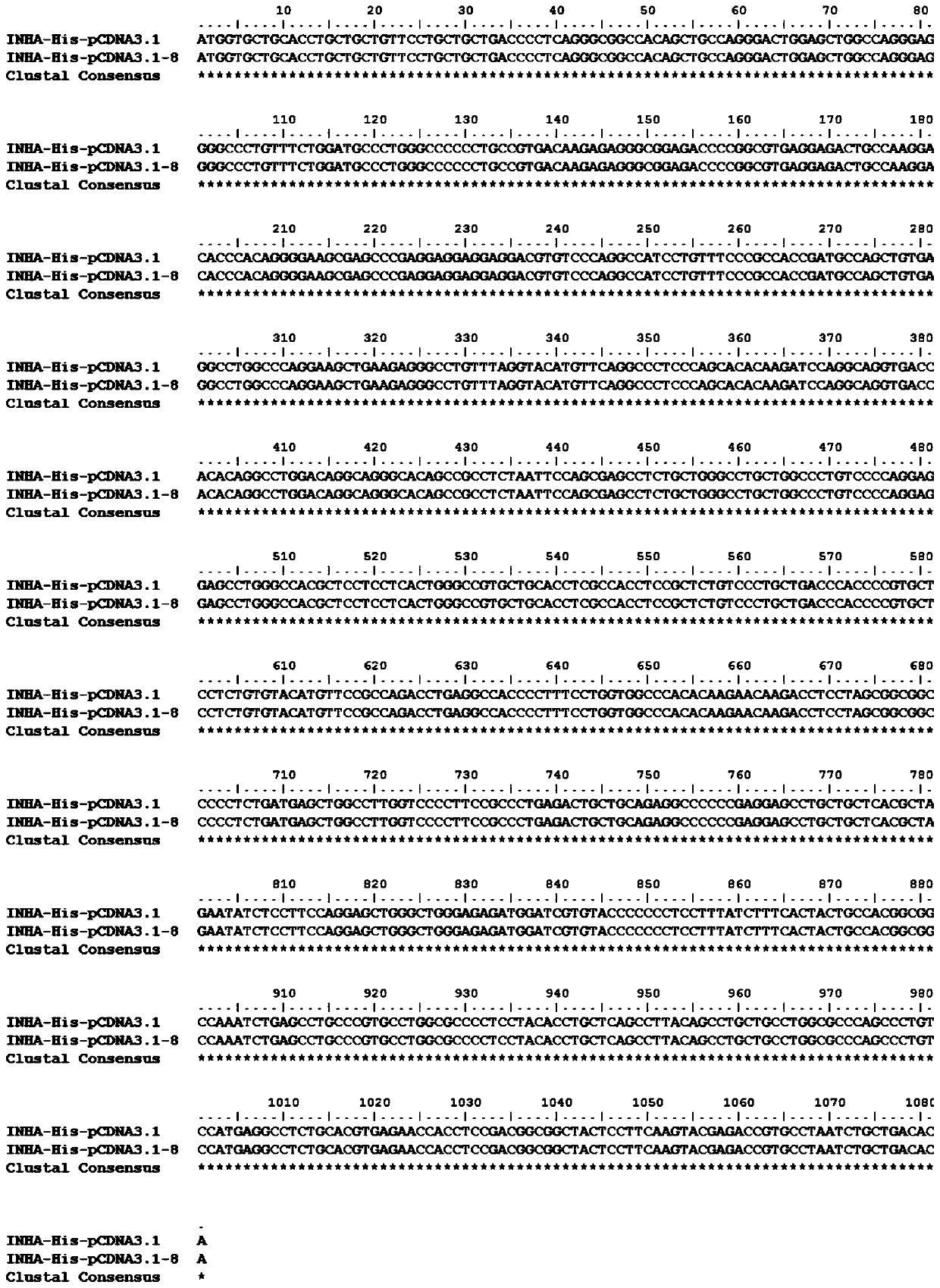
[0043] After analyzing and comparing the human inhibin A molecular sequence in Genbank, a large number of sequences in the nucleotide sequence of human inhibin A, such as signal peptide sequence, leader peptide sequence, enzyme cleavage site, G+C content and purification tag, etc. Information for sequence optimization design. The nucleotide sequence of the finally obtained human inhibin A is shown in Figure SEQ ID No.1, wherein the nucleotide sequence of the signal peptide nucleotide sequence is shown in SEQ ID No.2; the leader peptide nucleotide sequence The nucleotide sequence of the tag is shown in SEQ ID No.3; the nucleotide sequence of the purification tag is shown in SEQ ID No.4. The nucleotide sequence of the enzyme cutting site is NheI (the nucleotide sequence is gctagc) and XbaI (the nucleotide sequence is tctaga);
Embodiment 2
[0044] Embodiment 2: Construction of recombinant expression vector
[0045] This embodiment utilizes the nucleotide sequence of human inhibin A of embodiment 1 to construct a recombinant expression vector, the specific method is:
[0046] S1, designing primers for the nucleotide sequence of human inhibin A, specifically:
[0047] Upstream primer: 5'-ggagacccaagctggctagcgccgccaccatggtgctgcac-3';
[0048] Downstream primer: 5'-agcgggtttaaacgggccctctagattagtgatgatgatgatggtggctc-3';
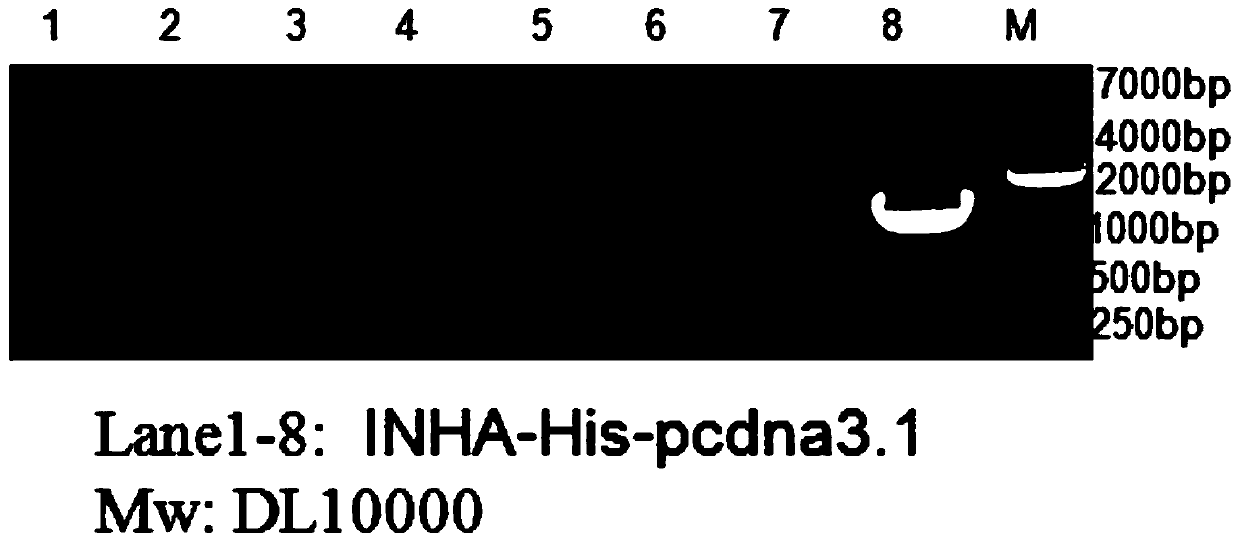
[0049] S2. Establish a 10 μL PCR amplification system: take 5 μL of 2×PrimerSTARMAX, 0.4 μL of upstream primers, 0.4 μL of downstream primers, and 0.5 μL of templates, and add to 10 μL with double distilled water; the reaction conditions are 95°C pre-denaturation for 10 seconds , annealing at 55°C for 5s, extension at 72°C for 10s, 30 cycles, and then holding at 16°C; the amplified product was detected by 1% agarose gel electrophoresis, and the target fragment was recovered;
[0050] At this time,...
Embodiment 3
[0061] Embodiment 3: the preparation method of human inhibin A
[0062] Transfect the recombinant expression vector prepared in Example 2 into HEK293 cells, culture with serum-free medium, collect the medium by centrifugation, then filter it with a suction filter, place it on ice to prepare the target protein, or place it at -20°C Save it for future use.
[0063] 1 day before transfection, cells were passed to 0.5 × 10 6 / ml, the specific method is:
[0064] P1. One day before transfection, take the cells and filter them with a cell mesh, count them, and make the number of cells reach 0.5×10 6 / ml; Take 30ml and transfer to a new 125ml shaker flask;
[0065] P2. On the day of transfection, prepare two 2ml centrifuge tubes, add 0.8ml OPTI-MEM medium respectively; add 30μg recombinant expression vector to one tube, add 60μg PEI to the other tube, and gently invert the centrifuge tube several times to mix evenly;
[0066] P3. Add the diluted PEI to the diluted recombinant exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com