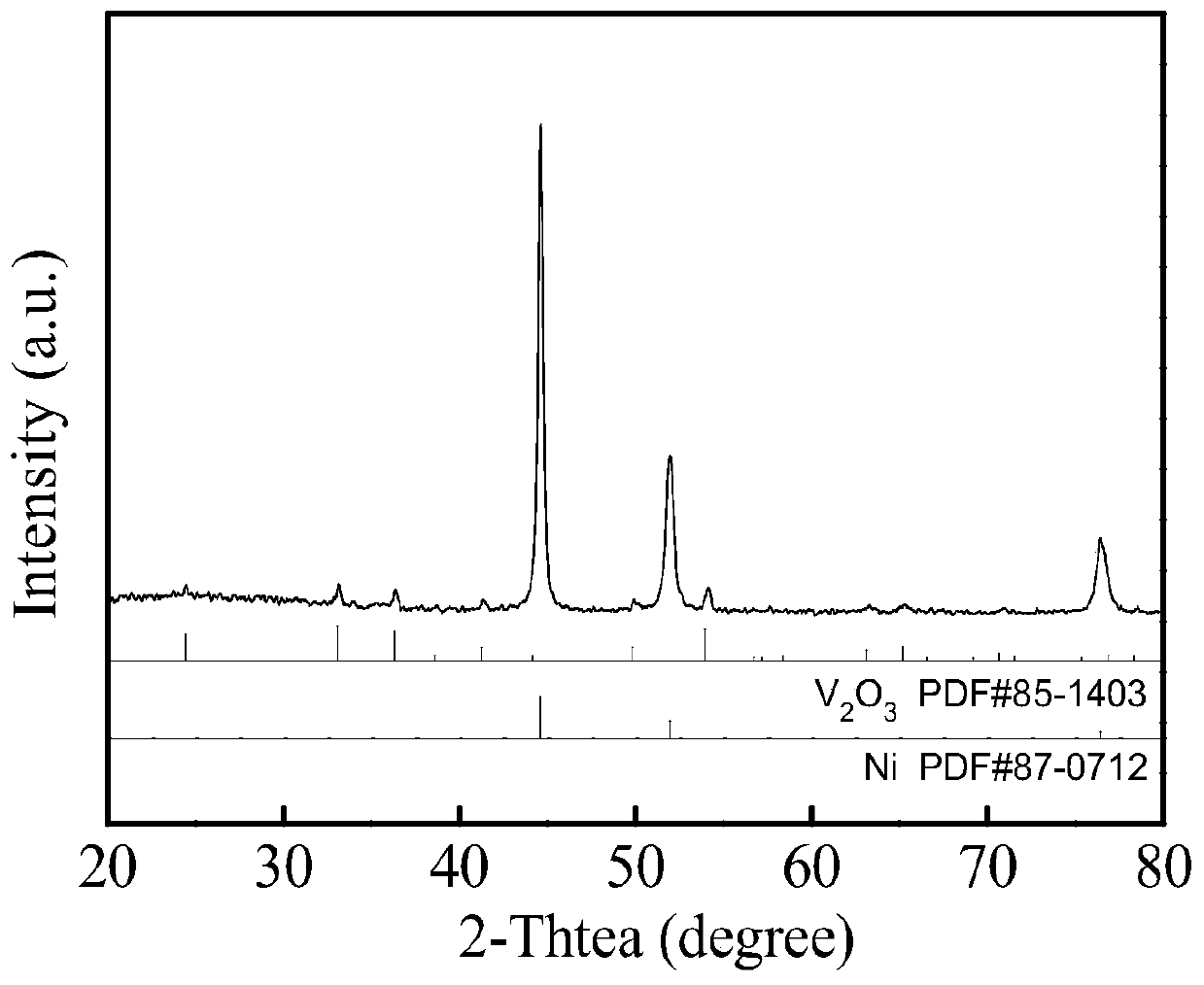
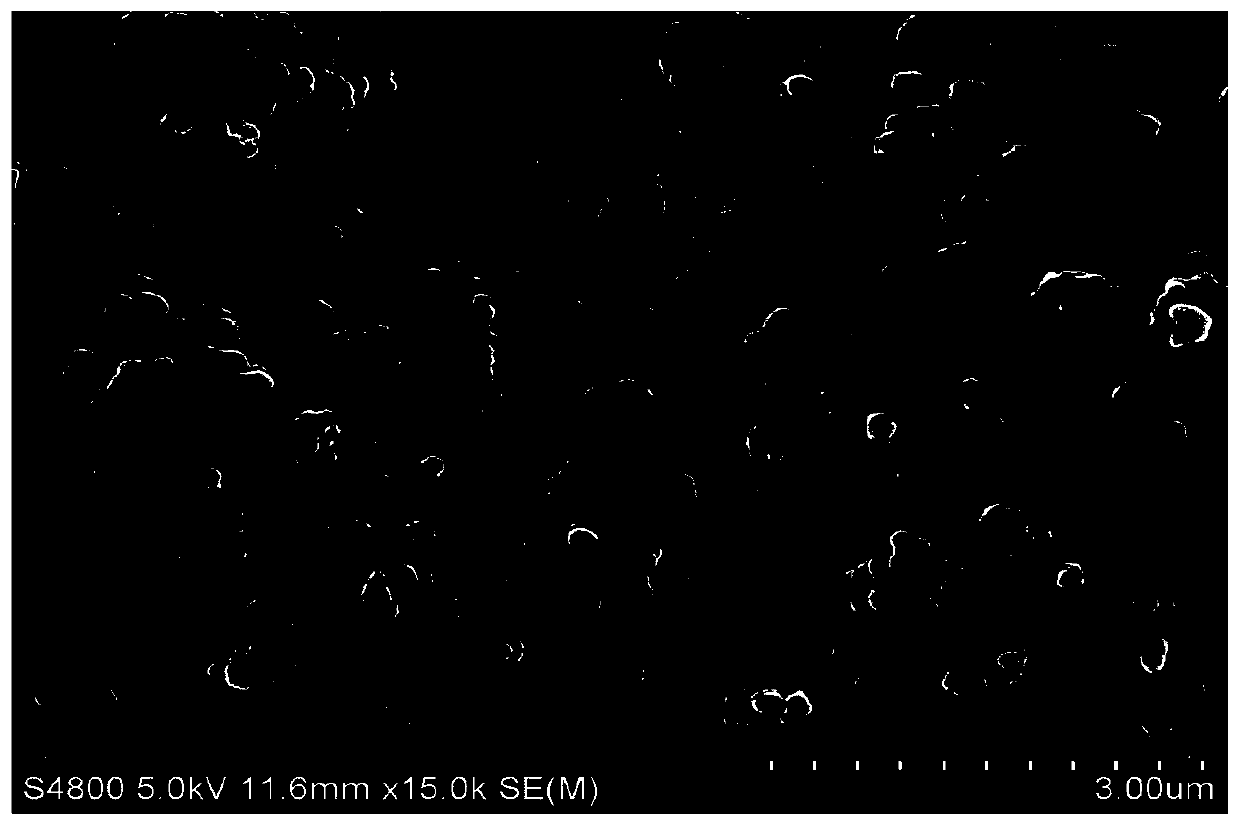
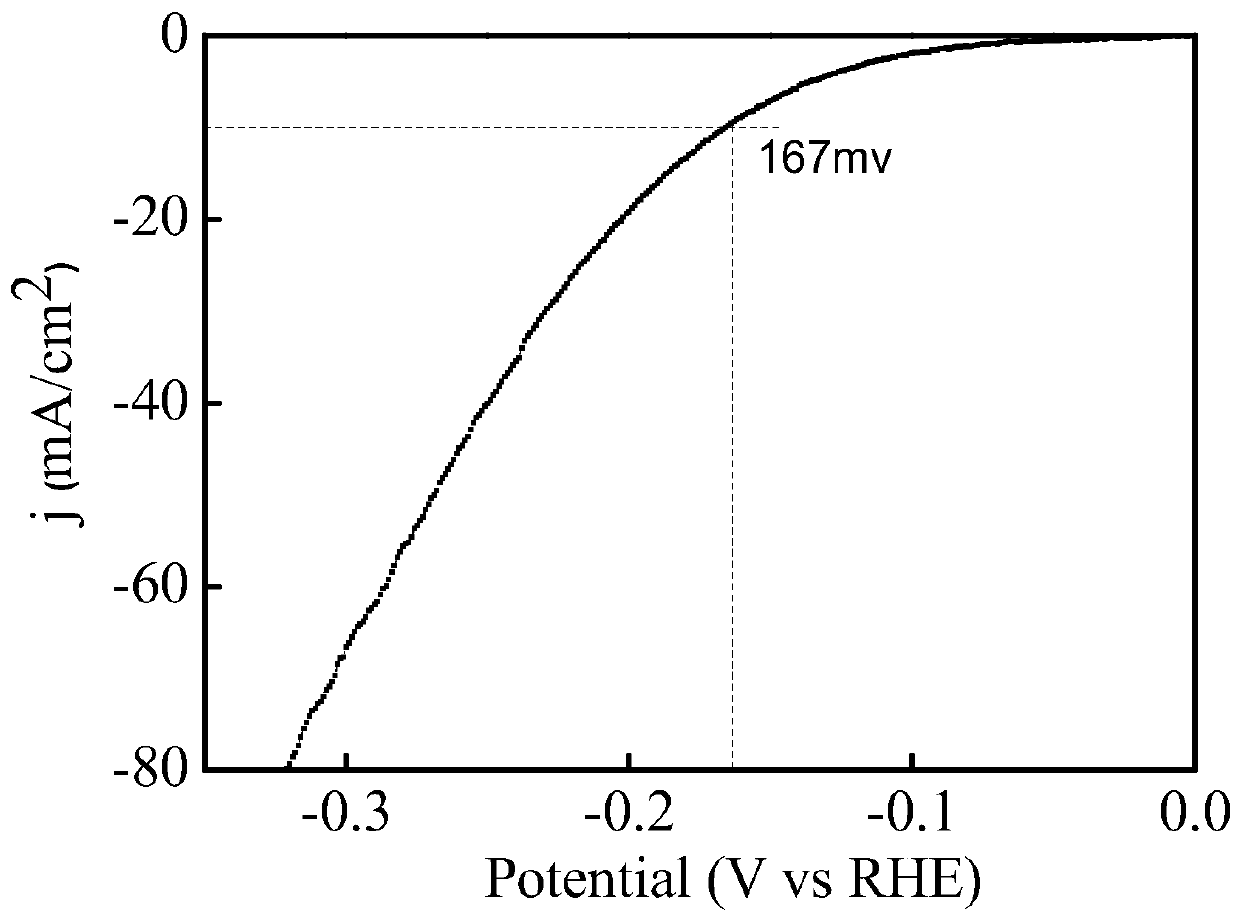
Preparation method of V2O3@Ni bifunctional composite electrocatalyst
An electrocatalyst and bifunctional technology, which is applied in the field of preparation of V2O3@Ni bifunctional composite electrocatalyst, can solve the problems of low efficiency, high energy consumption, easy mixing of impurities, etc., and achieves simple preparation process, improved specific surface area, and production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: The raw materials of urea, vanadium triisopropoxide oxide and nickel nitrate hexahydrate are mixed according to the mass ratio of 6:1:4 to form a mixture;
[0023] Step 2: Take 10mg of the mixture in step 1, grind it, pass through a 60-mesh sieve, pour it into the inner lining of the reaction kettle, add 50mL of ultra-pure water, put it on the mixer and stir for 20min, then install the inner lining in the outer kettle and place it in the In a homogeneous reactor;
[0024] Step 3: Heating to 180°C for hydrothermal reaction for 48 hours, and cooling to room temperature naturally after the reaction;
[0025] Step 4: Take out the product naturally cooled to room temperature, and collect the product after three times of alternate washing with water and three times of alcohol;
[0026] Step 5: Put the collected product into an oven, dry at 60°C for 8 hours, dry and grind to obtain the target product V 2 o 3 @Ni.
Embodiment 2
[0028] Step 1: The raw materials of urea, vanadium triisopropoxide oxide and nickel nitrate hexahydrate are mixed according to the mass ratio of 7:2:5 to form a mixture;
[0029] Step 2: Take 13mg of the mixture in step 1, grind it, pass it through a 60-mesh sieve, pour it into the lining of the reaction kettle, add 53ml of ultra-pure water and stir evenly, put it on the mixer and stir for 25min, then install the lining in the outer kettle and fix it. In a homogeneous reactor;
[0030] Step 3: heating to 200°C for hydrothermal reaction for 36 hours, and cooling to room temperature naturally after the reaction;
[0031] Step 4: Take out the product naturally cooled to room temperature, and collect the product after three times of alternate washing with water and three times of alcohol;
[0032] Step 5: Put the collected product into an oven, dry at 70°C for 7 hours, and grind to obtain the target product V 2 o 3 @Ni.
Embodiment 3
[0034] Step 1: The raw materials of urea, vanadium triisopropoxide oxide and nickel nitrate hexahydrate are mixed according to the mass ratio of 8:3:6 to form a mixture;
[0035] Step 2: Take 15mg of the mixture in step 1, grind it, pass it through a 60-mesh sieve, pour it into the inner lining of the reaction kettle, add 58mL of ultrapure water, put it on the mixer and stir for 28min, then install the inner lining in the outer kettle and fix it in the In a homogeneous reactor;
[0036] Step 3: heating to 190°C for hydrothermal reaction for 42 hours, and cooling to room temperature naturally after the reaction;
[0037] Step 4: Take out the product naturally cooled to room temperature, and collect the product after three times of alternate washing with water and three times of alcohol;
[0038] Step 5: Put the collected product into an oven, dry at 60°C for 6 hours, and grind to obtain the target product V 2 o 3 @Ni.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com