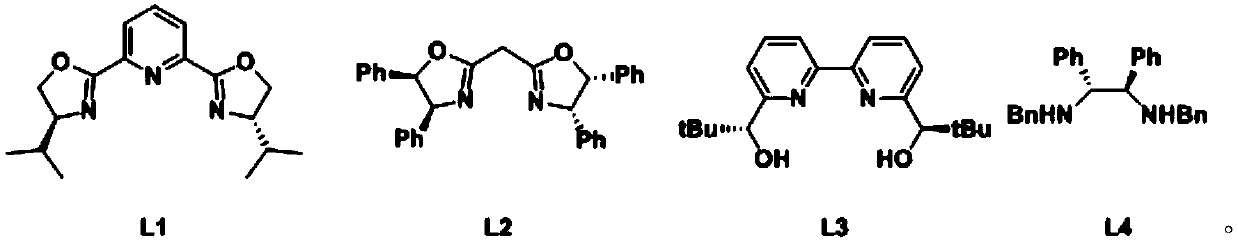
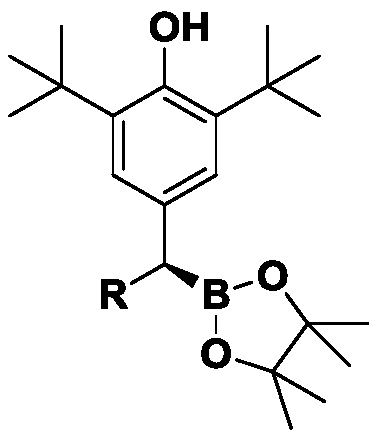
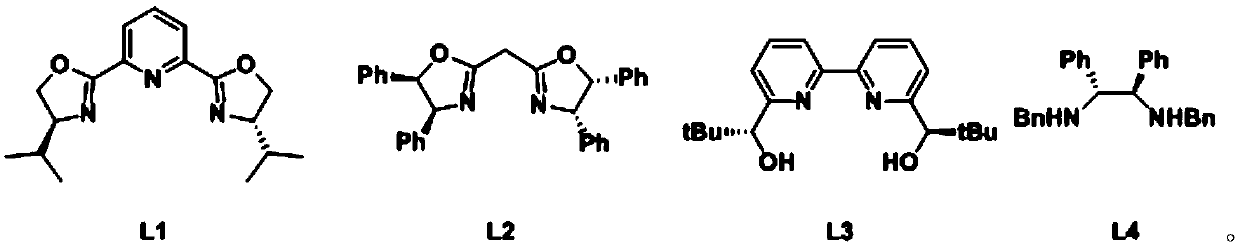
Method for preparing chiral organoboron compound
A compound and organoboron technology, which is used in the application fields of functionalized chitosan-supported copper catalyzed preparation of chiral organoboron compounds, synthesis of delta-hydroxy compounds and drug molecules for the treatment of diabetes, which can solve the problem of harming human health and environmental pollution. Large, metal residue and other problems, to achieve the effect of easy method, wide application and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation method of compound III-1, its steps are:
[0084]
[0085] A. Add functionalized chitosan-supported copper chloride catalytic material (HBCS@CuCl 2 ) 5mg and ligand L3 (3.0mg, 0.012mmol, 6mol%), add 2.5 mL of water, and stir at room temperature (20 or 22 or 24 or 25°C, the same below) for 1 hour;
[0086] B. To the above system, add starting material I-1 (58.9mg, 0.2mmol) and pinacol diborate (B 2 (pin) 2 ) (60.9mg, 0.24mmol);
[0087] C, the whole reaction system was stirred at room temperature for 12 hours to react;
[0088] D, after the reaction finishes, filter the whole reaction system, wash with tetrahydrofuran 3mL, directly add sodium perborate tetrahydrate (244mg, 0.8mmol) in the residue, and the whole system is stirred at room temperature for 4 hours;
[0089] E. Add ethyl acetate 3mL to the above system for dilution, extract with ethyl acetate (3×10mL), separate the organic phase, and use anhydrous Na 2 SO 4 Dry, filter and remove solv...
Embodiment 2
[0093] The preparation method of compound III-2, its steps are:
[0094]
[0095] A. Add functionalized chitosan-supported copper chloride catalytic material (HBCS@CuCl 2 ) 5mg and ligand L3 (3.0mg, 0.012mmol, 6mol%), add 2.5mL of water, and stir at room temperature (20 or 22 or 24 or 25°C, the same below) for 1 hour;
[0096] B, in above-mentioned system, successively add starting material I-2 (65.8mg, 0.2mmol) and biboronic acid pinacol ester (B 2 (pin) 2 ) (60.9mg, 0.24mmol);
[0097] C, the whole reaction system was stirred at room temperature for 12 hours to react;
[0098] D, after the reaction finishes, filter the whole reaction system, wash with tetrahydrofuran 3mL, directly add sodium perborate tetrahydrate (244mg, 0.8mmol) in the residue, and the whole system is stirred at room temperature for 4 hours;
[0099] E. Add 3 mL of ethyl acetate to the above system to dilute, extract with ethyl acetate (3×10 mL), separate the organic phase, and use anhydrous Na 2 S...
Embodiment 3
[0104] Preparation of compound III-3:
[0105]
[0106] A. Add functionalized chitosan-supported copper chloride catalytic material (HBCS@CuCl 2 ) 5mg and ligand L3 (3.0mg, 0.012mmol, 6mol%), add 2.5mL of water, and stir at room temperature (20 or 22 or 24 or 25°C, the same below) for 1 hour;
[0107] B, in above-mentioned system, successively add starting material I-3 (74.7mg, 0.2mmol) and biboronic acid pinacol ester (B 2 (pin) 2 ) (60.9mg, 0.24mmol);
[0108] C, the whole reaction system was stirred at room temperature for 12 hours to react;
[0109] D, after the reaction finishes, filter the whole reaction system, wash with tetrahydrofuran 3mL, directly add sodium perborate tetrahydrate (244mg, 0.8mmol) in the residue, and the whole system is stirred at room temperature for 4 hours;
[0110] E. Add 3 mL of ethyl acetate to the above system to dilute, extract with ethyl acetate (3×10 mL), separate the organic phase, and use anhydrous Na 2 SO 4 Dry, filter and remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com