Furanyl copolyester and its preparation method
A technology of furan-based copolyester and furandicarboxylic acid, applied in the polymer field, can solve the problems of low glass transition temperature, unfavorable product processing, and difficulty in meeting the requirements, and achieve the effects of improving transparency, not easy to crack, and slowing down thermal crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of furyl copolyester provided by the invention comprises:
[0029] (1) 2,5-furandicarboxylic acid or its esterified product, bis[4-(2-hydroxyethoxy)phenyl]sulfone, cyclohexanedimethanol, aliphatic hydrocarbon dibasic alcohol and esterification catalyst are mixed, and Carry out esterification reaction, obtain the first intermediate product, wherein, the structural formula of described two [4-(2-hydroxyethoxy) phenyl] sulfone is as shown in formula (2):
[0030]
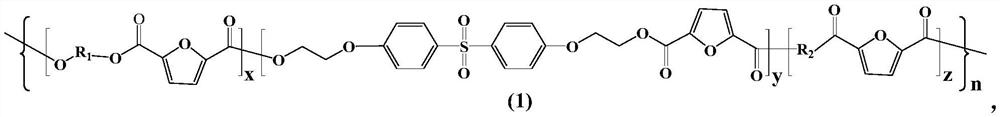
[0031] (2) The first intermediate product is subjected to a polycondensation reaction to obtain a furyl copolyester, wherein the structural formula of the furyl copolyester is as shown in formula (1):
[0032]
[0033] Among them, x, y, and z are all integers from 1 to 20, n is an integer from 10 to 100, and R 1 is a structural unit corresponding to aliphatic dihydric alcohol, the R 1 The chemical structural formula is -(CH 2 ) m -, m is an integer from 2 to 10, R 2 is a structu...
Embodiment 1
[0070] Dimethyl 2,5-furandicarboxylate 0.25mol, bis[4-(2-hydroxyethoxy)phenyl]sulfone 0.125mol, 1,4-cyclohexanedimethanol 0.075mol and 1,4-butane 0.2 mol of diol was added into the polymerization reactor, and then 0.15% of tetrabutyl titanate based on the molar amount of dimethyl 2,5-furandicarboxylate was added. Under an inert atmosphere, react at 180° C. for 4 hours to obtain the first intermediate product.
[0071] Add 0.12% triphenyl phosphate based on the molar amount of dimethyl 2,5-furandicarboxylate and 0.1% antioxidant-1010 based on the theoretical polymer yield to the first intermediate product, and gradually reduce the vacuum to 100Pa Thereafter, gradually raise the temperature to 240° C., and react for 4.5 hours to obtain a furyl copolyester.
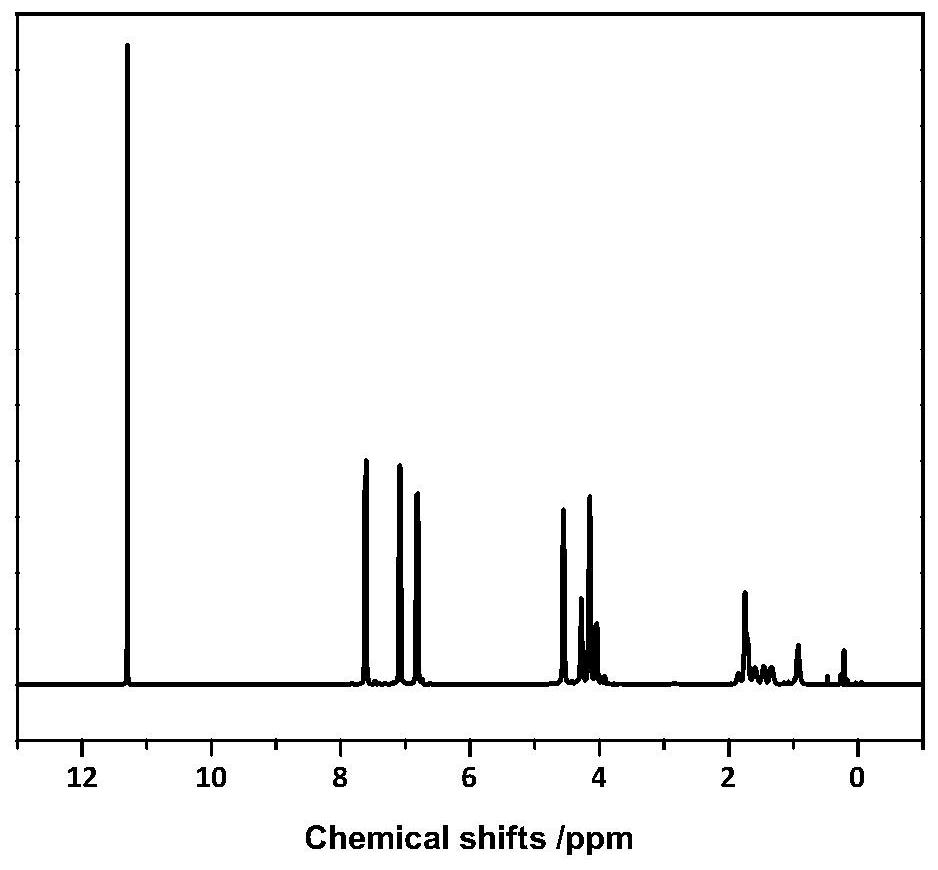
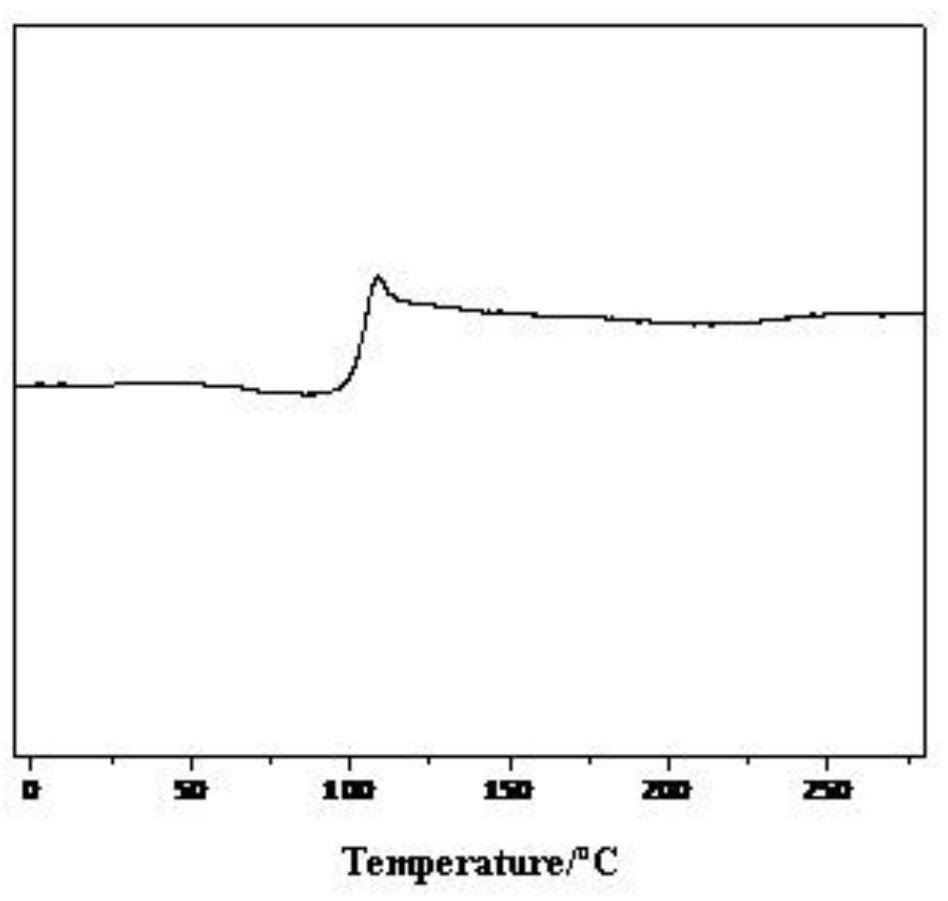
[0072]After testing, the relative number average molecular weight of the furyl-containing copolyester is 28500g / mol, and the relative weight average molecular weight is 47800g / mol. The furyl copolyester's 1 H-NMR such as ...
Embodiment 2
[0074] Dimethyl 2,5-furandicarboxylate 0.25mol, bis[4-(2-hydroxyethoxy)phenyl]sulfone 0.125mol, 1,4-cyclohexanedimethanol 0.05mol and 1,4-butane 0.225 mol of diol was added into the polymerization reactor, and then 0.15% of tetrabutyl titanate based on the molar amount of dimethyl 2,5-furandicarboxylate was added. Under an inert atmosphere, react at 180° C. for 4 hours to obtain the first intermediate product.
[0075] Add 0.12% triphenyl phosphate based on the molar amount of dimethyl 2,5-furandicarboxylate and 0.1% antioxidant-1010 based on the theoretical polymer yield to the first intermediate product, and gradually reduce the vacuum to 100Pa Then, gradually raise the temperature to 235° C., and react for 4 hours to obtain furyl copolyester.
[0076] After testing, the relative number average molecular weight of the furyl-containing copolyester is 31800g / mol, and the relative weight average molecular weight is 54300g / mol. After detection, the molar ratio of bis[4-(2-hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Relative number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com