Sulfated xylan and preparation method and application sulfated xylan to preparation of antioxidant drugs
A xylan and sulfation technology, applied in the field of xylan modification, can solve the problems of low degree of substitution, cumbersome separation process, complex polysaccharide structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation method of sulfated xylan.
[0034] Weigh 100mg of xylan, dissolve it in 50mL N,N-dimethylformamide in a conical flask, stir with a magnetic stirrer and raise the temperature to 80°C, add 2.5g of sulfamic acid, stir for 1 hour, stop in ice bath For the reaction, the pH was adjusted to neutral with 20% sodium hydroxide solution, dialyzed in running water for 2 days, and the sulfated xylan sample was obtained after freeze-drying.
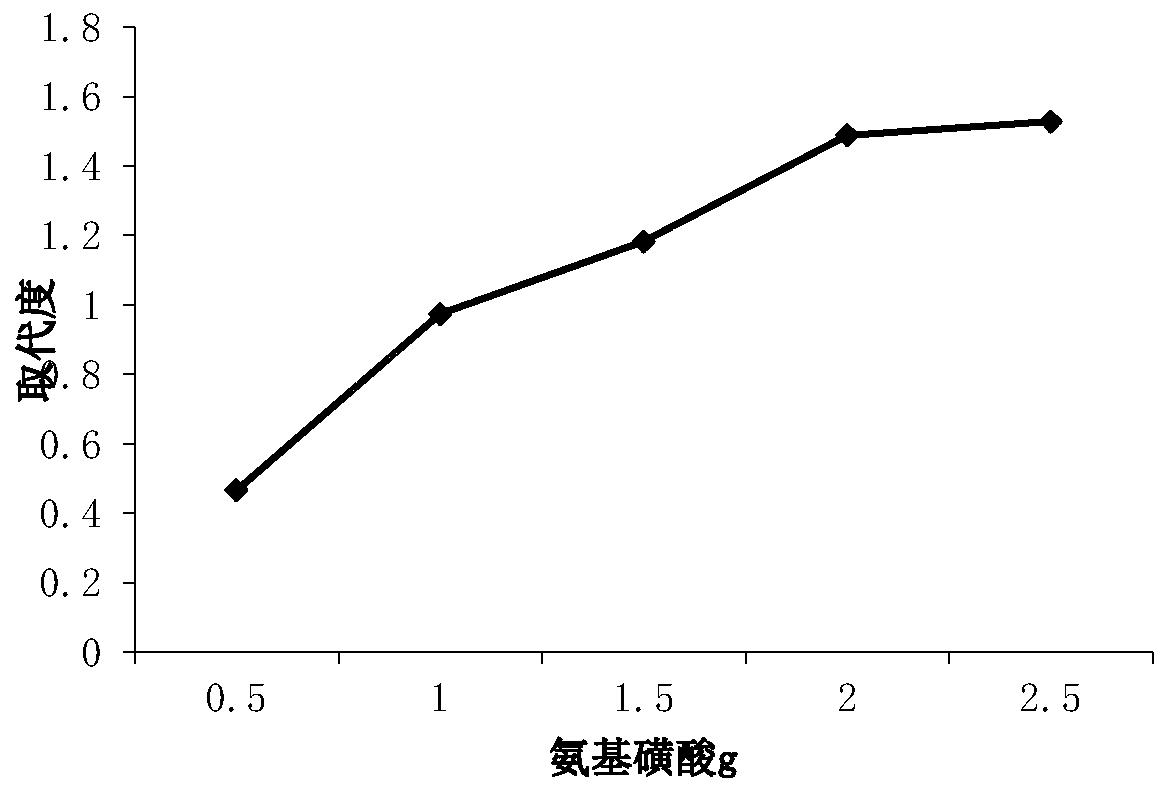
[0035] 1. Effect of different dosages of sulfamic acid on the degree of sulfation substitution of xylan
[0036] Set the amount of sulfamic acid to 0.5g, 1g, 1.5g, 2.0g, 2.5g, and study the effect of different amounts of sulfamic acid on the degree of sulfation substitution of xylan.
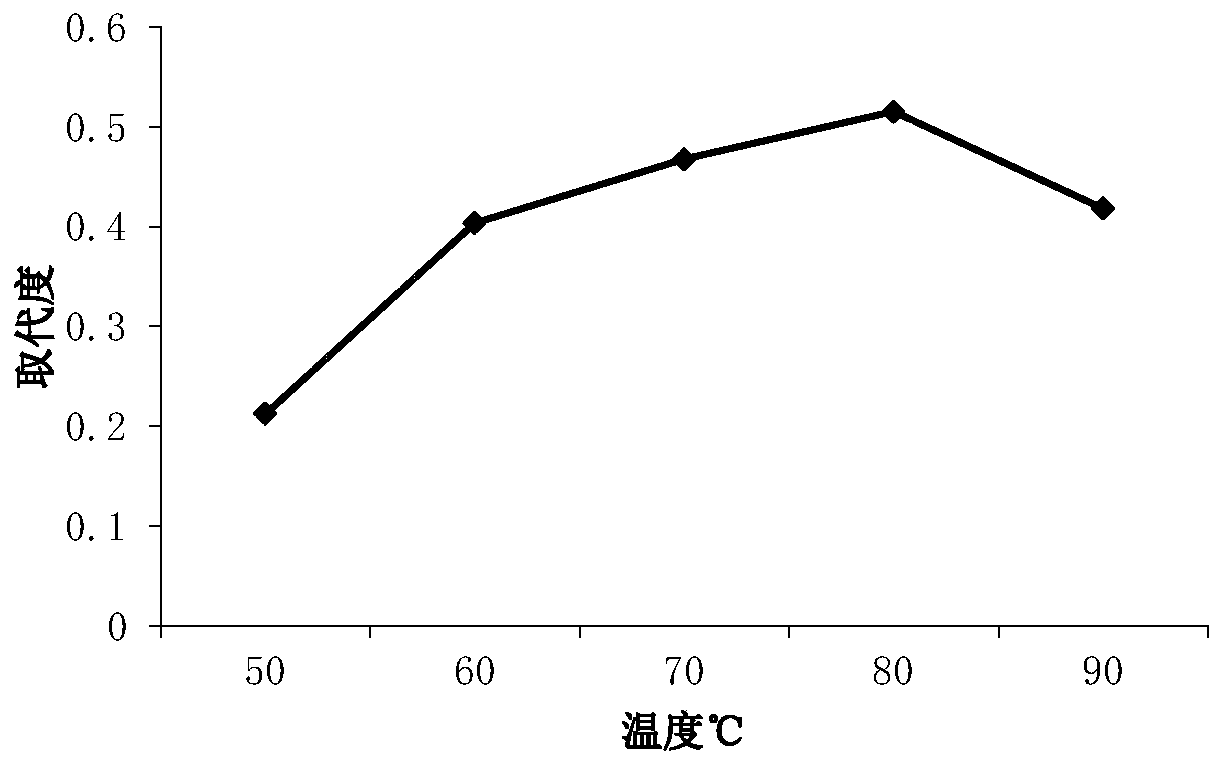
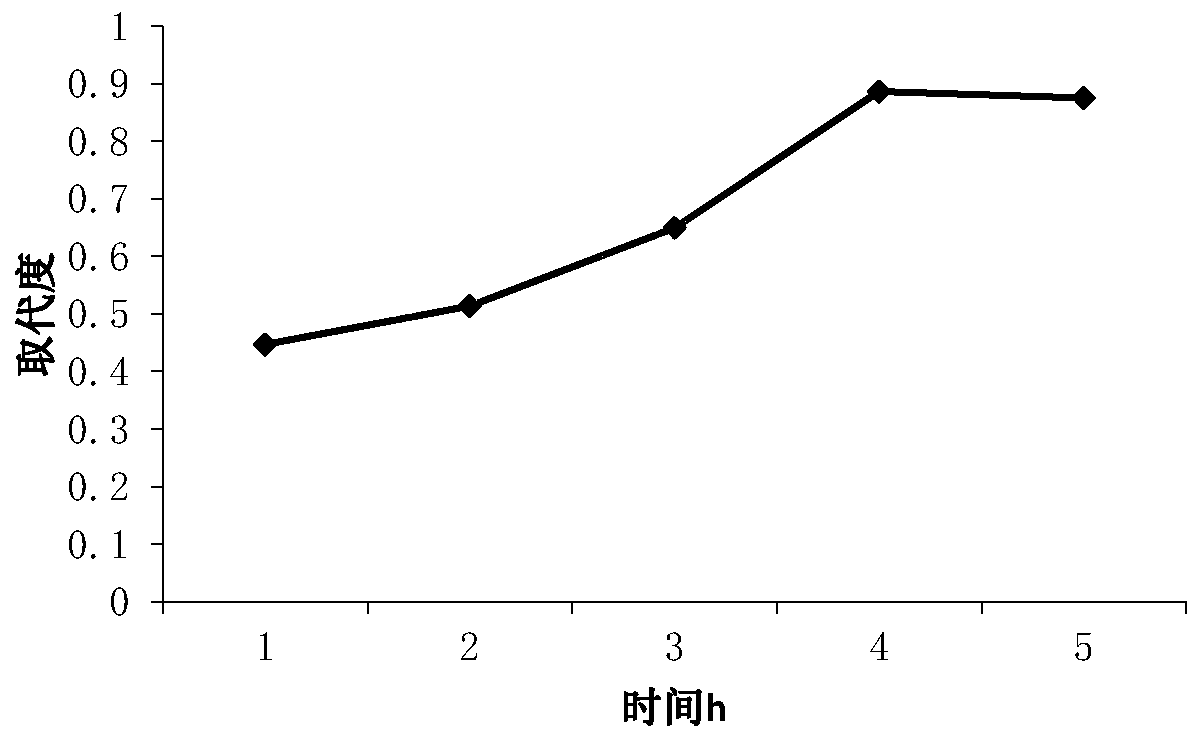
[0037] Such as figure 1 As shown, the results show that when the amount of sulfamic acid added is 0.5-1.5g, the degree of substitution changes gently, and there is the highest peak at 2.5g, that is, the degree of substitution is 1.53, and the degree ...
Embodiment 2
[0048] Sulfated xylan was prepared through the optimal reaction conditions obtained in Example 1, and analyzed by infrared spectroscopy.
[0049] Specifically: weigh 2 mg of xylan and sulfated xylan prepared under optimal conditions respectively, add 100 mg of dry KBr into an agate mortar, grind evenly, and press into tablets. Carry out infrared spectrum scanning, the scanning range is 4000~400 cm-1 .
[0050] Such as Figure 5 As shown, the result shows: at 1197.63cm -1 , the absorption peak increased significantly, indicating that the sulfate radical increased, 831.20cm -1 is the characteristic absorption peak of sulfate ester bonds, indicating that sulfate groups have been linked to polysaccharide chains, and xylan has successfully prepared sulfated xylan.
Embodiment 3
[0052] Determination of degree of substitution of sulfated xylan
[0053] Weigh 3mg of the sample into a test tube, add 3mL of 1mol / L HCl, stopper, hydrolyze in a constant temperature water bath at 100°C for 6 hours, evaporate the contents to dryness at 60°C with an evaporating dish, and redissolve in 2mL of deionized water to obtain the sample hydrolysis liquid. Take 0.4mL of hydrolyzate, make up to 1.6mL with deionized water, add 8% trichloroacetic acid solution, BaCl 2 - 1.4mL each of the gelatin solutions, mix well and let stand for 15min, measure the absorbance A1 at a wavelength of 360nm.
[0054] Control group: the operation of the control group is the same as above, with 1.4mL of gelatin solution instead of BaCl 2 - Gelatin solution. The absorbance value A2 was measured at a wavelength of 360 nm.
[0055] The required absorbance A0=A1-A2, the purpose of A1-A2 is to eliminate the influence of the self-absorbing substances contained in the hydrolyzate. The quality o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com