Treatment of type 2 diabetes mellitus with gene modified MSCs
A gene and gene-encoding technology, applied in the preparation method and application, as well as in the field of drugs for treating type 2 diabetes and recombinant stem cells, can solve problems such as inability to treat diabetes, and achieve the effects of improving sensitivity, long-term action, and reducing blood sugar and blood lipid levels Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
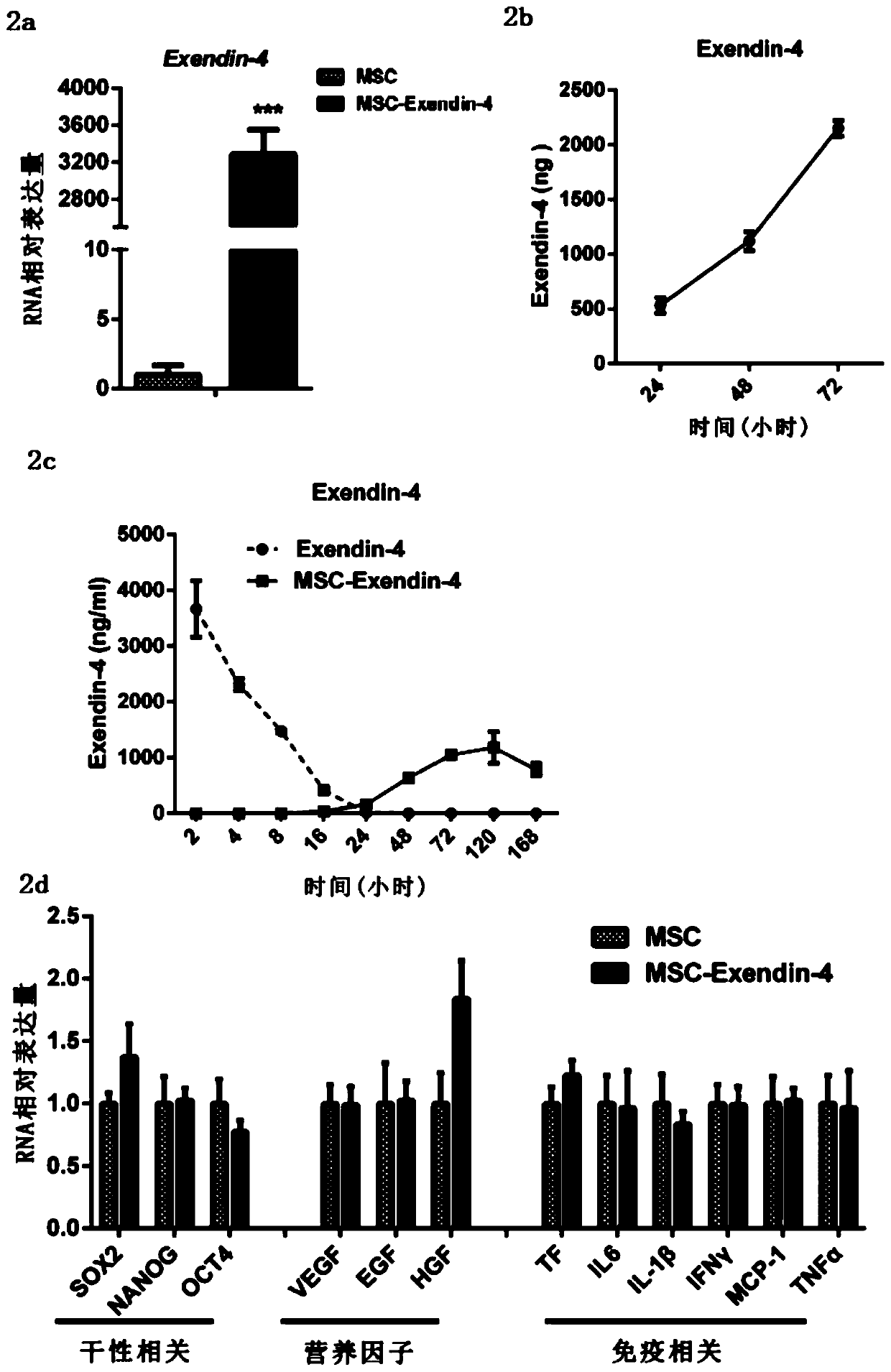
[0047] Embodiment 1 provides a method for preparing recombinant mesenchymal stem cells, comprising:
[0048] 1. Isolation and identification of adipose-derived mesenchymal stem cells
[0049] Human adipose-derived mesenchymal stem cells and adipose tissue were provided by the Plastic Surgery Department of Peking Union Medical College Hospital. The samples were obtained and volunteers signed an informed consent. The fat donors are healthy people aged 25-35 without HIV, HBV and HCV infection. The fat sample is the discarded fat during liposuction, which is sucked out and stored in a sterile, airtight disposable drainage bag, stored at low temperature and transported to the laboratory for the isolation of primary adipose-derived mesenchymal stem cells. Prepare collagenase type II 0.2%, a large amount of HBSS+2% P / S, wash the fat with HBSS equal to the volume of fat for 5 times, and wash away the blood components. Add collagenase solution equal to the volume of fat in each tube,...
Embodiment 2
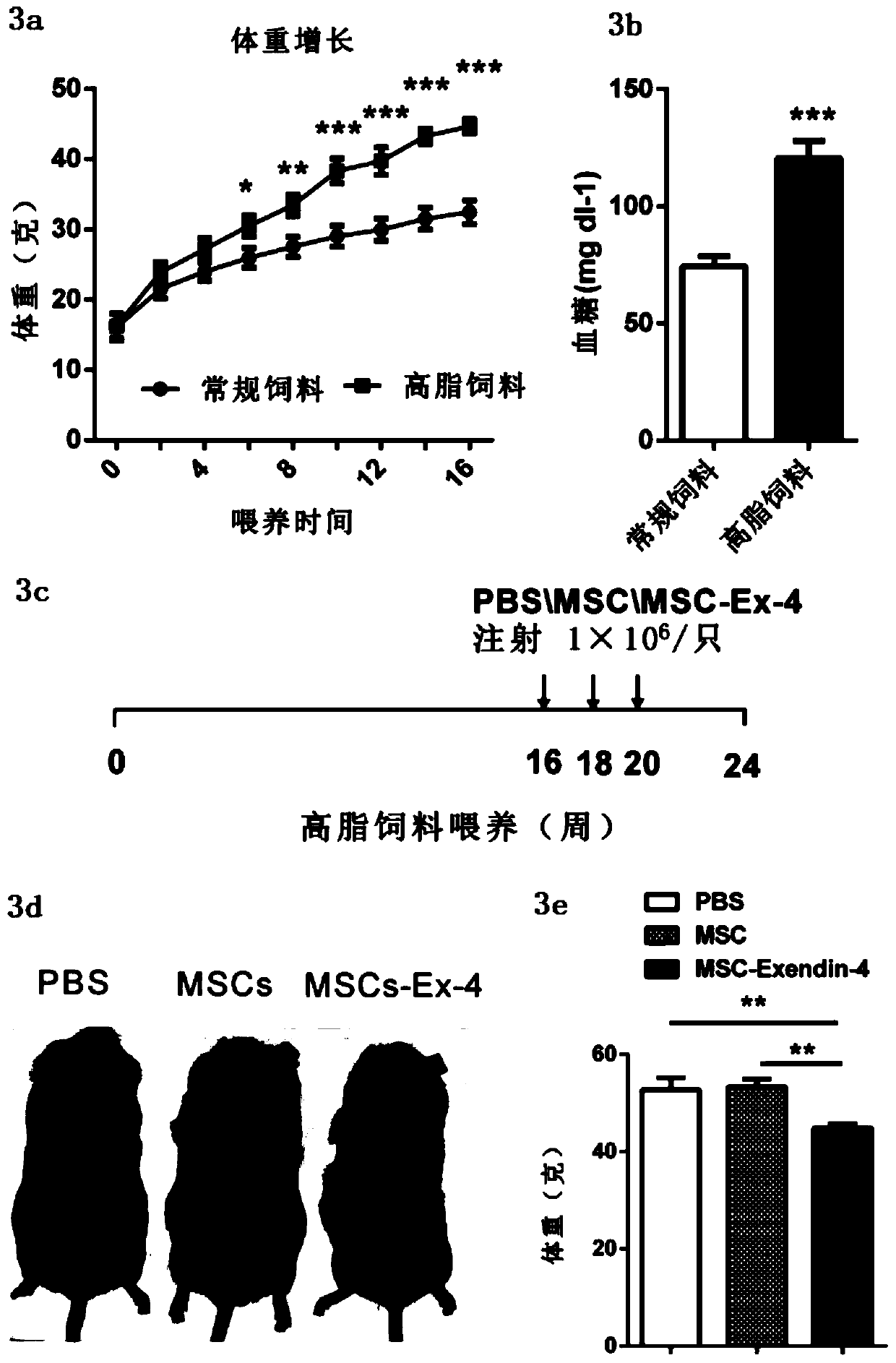
[0064] Example 2 Determination of biochemical indicators
[0065] The obese diabetic mouse model was established by feeding the mice with high fat for 16 weeks, and the blood glucose concentration in the tail vein blood was detected with a blood glucose meter. Blood samples were collected by heart blood collection, put into EDTA anticoagulant tubes, and centrifuged at 2000 rpm at 4°C for 15 min. The supernatant was taken for the detection of blood indicators. 4 μl of plasma was taken to detect the insulin content with a kit (Mercodia, 10-1247-01). 10 μl of plasma was taken to detect triglyceride content with a kit (Sigma, TR0100). Take 20 microliters and send it to the hospital for liver function testing.
[0066] By feeding mice with high fat for 16 weeks, an obese diabetic mouse model was established, and the weight of the mice increased significantly, reaching obesity, such as image 3 as shown in a. Detect that the blood glucose level of mice fed with high-fat feed is...
Embodiment 3
[0070] Example 3 Detection of MSCs survival and distribution in vivo
[0071] The survival and distribution of MSCs in vivo are very important for their therapeutic effects. Therefore, using Akaluc luciferase to react with luciferin to generate bioluminescent signals can detect the position, distribution and number of cells in the body. Taking MSCs cells as an example, by constructing a lentivirus containing the gene encoding Akaluc luciferase, and infecting MSCs cells with the lentivirus, MSC-Akaluc cells (that is, MSC cells capable of expressing Akaluc luciferase) can be obtained. Express luciferase, which produces bioluminescence when Akaluc substrate is added, by The strength of the signal captured by Lumina II reflects the amount of cells.
[0072] Therefore, we injected MSC-Akaluc cells into mice fed with conventional diet and high-fat diet, and injected Akaluc substrate intraperitoneally, and observed the bioluminescent signal 6 hours after injection, and found that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com