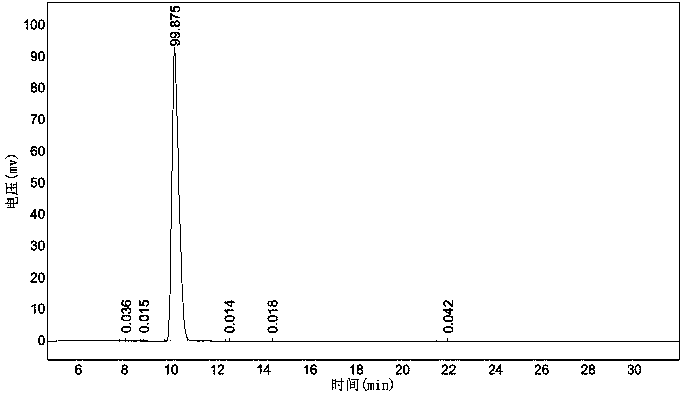
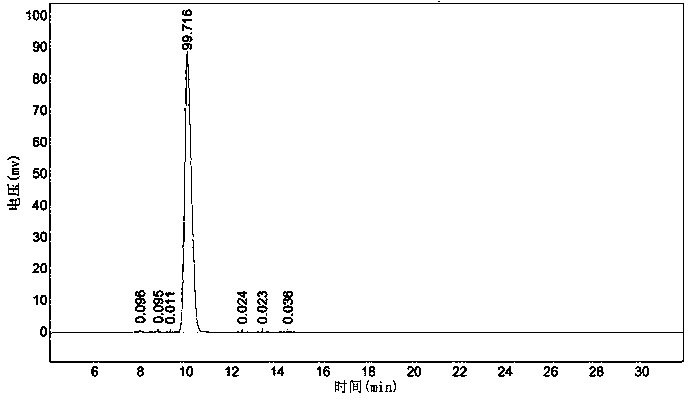
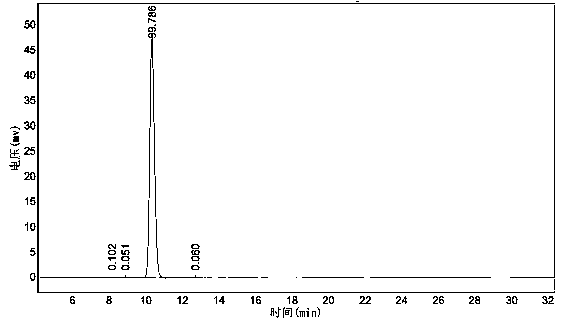
Catalytic synthesis method of ursodeoxycholic acid (UDCA)
A technology of ursodeoxycholic acid and synthetic methods, applied in the direction of microorganism-based methods, biochemical equipment and methods, oxidoreductase, etc., can solve problems such as low conversion rate, poor product quality, long reaction cycle, etc., and achieve reaction Efficiency improvement, elimination of use, and reduction of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0034] 1. Preparation of 7-β dehydroxylase compound glucose dehydrogenase solution
[0035] The 7-beta dehydroxylase compound glucose dehydrogenase gene was recombined into engineered Escherichia coli, cultured at 37°C for 36 hours, and the recombined Escherichia coli was collected by centrifugation at 8000 rpm;
[0036] Take 400g of Escherichia coli collected by centrifugation and resuspend in 1600ml, 0.05M phosphate buffer, and homogeneously crush twice under 50MPa pressure;
[0037] Take 1000ml of the above solution, centrifuge at 8000 rpm for 20 minutes, collect 946ml of supernatant, and obtain the 7-beta dehydroxylase composite glucose dehydrogenase solution used in the catalytic reaction of the present invention.
[0038] 2. Preparation of 7-KLCA solution
[0039] Take 17.5g of 7-KLCA solid, add it into 92ml deionized water, stir evenly, then gradually add 40% sodium hydroxide solution dropwise under stirring condition until the solution is just dissolved, and the pH of...
Embodiment 2
[0049] 1. Preparation of 7-β dehydroxylase compound glucose dehydrogenase solution
[0050] The 7-beta dehydroxylase compound glucose dehydrogenase gene was recombined into engineered Escherichia coli, cultured at 37°C for 36 hours, and the recombined Escherichia coli was collected by centrifugation at 8000 rpm;
[0051] Take 400g of Escherichia coli collected by centrifugation and resuspend in 1600ml, 0.05M phosphate buffer, and homogeneously crush twice under 50MPa pressure;
[0052] Take 1000ml of the above solution, centrifuge at 8000 rpm for 20 minutes, collect 946ml of supernatant, and obtain the 7-beta dehydroxylase composite glucose dehydrogenase solution used in the catalytic reaction of the present invention.
[0053] 2. Preparation of 7-KLCA solution
[0054] Take 10g of 7-KLCA solid, add it into 60ml deionized water, stir evenly, then gradually add 40% sodium hydroxide solution dropwise under stirring condition until the solution is just dissolved, and the pH of t...
Embodiment 3
[0065] 1. Preparation of 7-β dehydroxylase compound glucose dehydrogenase solution
[0066] The 7-beta dehydroxylase compound glucose dehydrogenase gene was recombined into engineered Escherichia coli, cultured at 37°C for 36 hours, and the recombined Escherichia coli was collected by centrifugation at 8000 rpm;
[0067] Take 400g of Escherichia coli collected by centrifugation and resuspend in 1600ml, 0.05M phosphate buffer, and homogeneously crush twice under 50MPa pressure;
[0068] Take 1000ml of the above solution, centrifuge at 8000 rpm for 20 minutes, collect 946ml of supernatant, and obtain the 7-beta dehydroxylase composite glucose dehydrogenase solution used in the catalytic reaction of the present invention.
[0069] 2. Preparation of 7-KLCA solution
[0070] Take 16g of 7-KLCA solid, add it to 90ml deionized water, stir evenly, then gradually add 40% sodium hydroxide solution dropwise under stirring condition until the solution is just dissolved, and the pH of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com