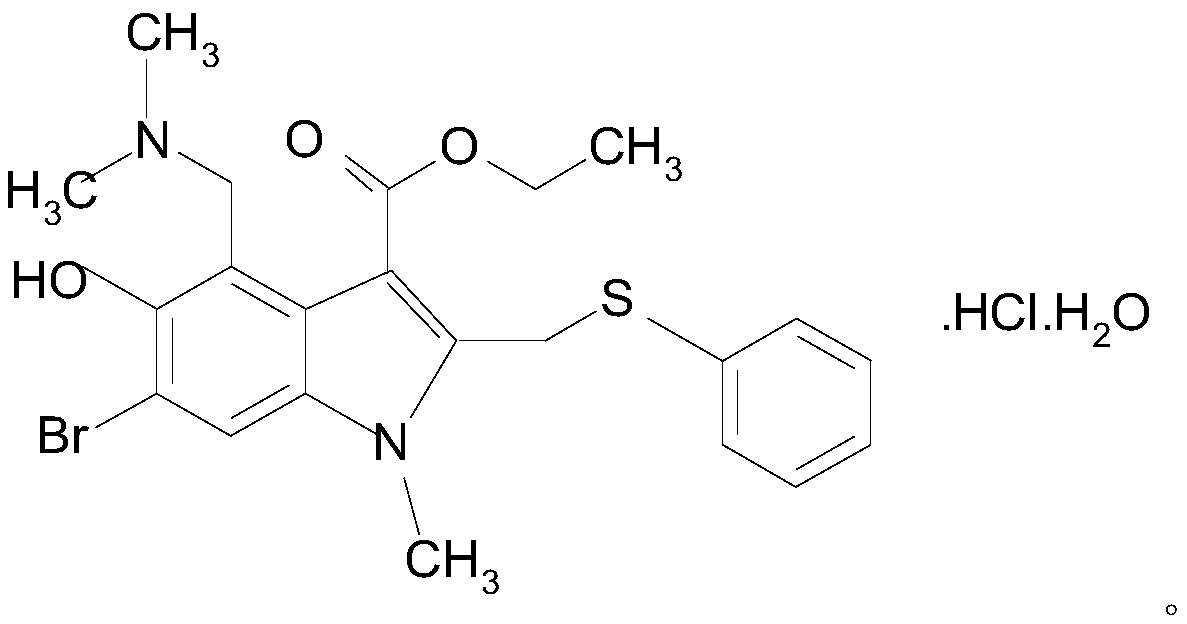
Arbidol hydrochloride injection preparation and preparation method thereof
A technology for arbidol hydrochloride and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of low absolute bioavailability, short duration of drug effect, Short biological half-life and other issues, to achieve the effect of being convenient for clinical use, prolonging the administration time, and lasting for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
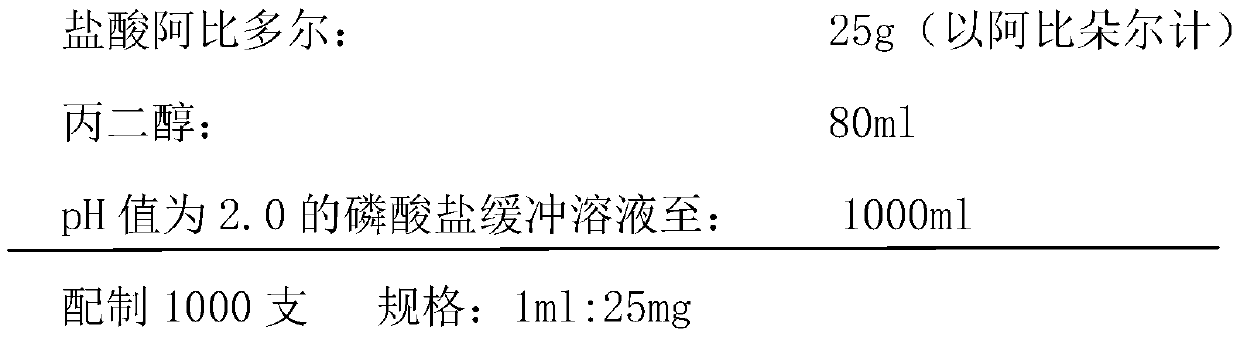
[0031] 1) Prescription composition (1000 prescriptions)
[0032]
[0033] 2) Preparation process:
[0034] (1) Measure the phosphate buffer solution with a pH value of 2.0 of 30% of the prescription quantity in the liquid mixing tank;
[0035] (2) Add the propylene glycol of prescription quantity and make stirring, then slowly and evenly add the Arbidol hydrochloride of prescription quantity and make it fully dissolve;
[0036] (3) Adjust the pH value to 2.3, add 0.1% (w / v) activated carbon and stir evenly, keep the temperature at 60°C±5°C, and adsorb for 30 minutes;
[0037] (4) Decarburization, after detecting the content and pH of the filtrate, add a phosphate buffer solution with a pH value of 2.0, filter the solution through a two-stage 0.22-micron microporous membrane, and then fill it in a container that has been sterilized at >350°C for >5 minutes ampoule.
[0038] (5) Sterilization, water bath sterilization at 115°C for 30 minutes.
Embodiment 2
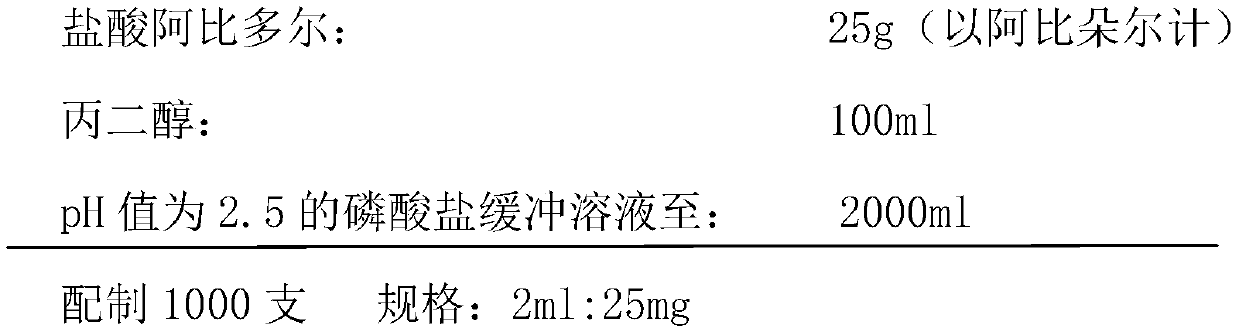
[0040] 1) Prescription composition (1000 prescriptions)
[0041]
[0042] 2) Preparation process:
[0043] (1) Measure 30% of the prescription amount of phosphate buffer solution with a pH value of 2.5 in the liquid mixing tank;
[0044] (2) Add the propylene glycol of prescription quantity and make stirring, then slowly and evenly add the Arbidol hydrochloride of prescription quantity and make it fully dissolve;
[0045] (3) Adjust the pH value to 2.7, add 0.1% (w / v) activated carbon, stir evenly, and keep the temperature at 60°C±5°C for 30 minutes;
[0046] (4) Decarburization, after detecting the content and pH of the filtrate, add a phosphate buffer solution with a pH value of 2.5, filter the solution through a two-stage 0.22 micron microporous membrane, and then fill it in a container that has been sterilized at >350°C for >5 minutes ampoule.
[0047] (5) Sterilization, water bath sterilization at 115°C for 30 minutes.
Embodiment 3
[0049] 1) Prescription composition (1000 prescriptions)
[0050]
[0051] 2) Preparation process:
[0052] (1) Measure the phosphate buffer solution with a pH value of 2.0 of 30% of the prescription quantity in the liquid mixing tank;
[0053] (2) Add the propylene glycol of prescription quantity and make stirring, then slowly and evenly add the Arbidol hydrochloride of prescription quantity and make it fully dissolve;
[0054] (3) Adjust the pH value to 2.2, add 0.1% (w / v) activated carbon, stir evenly, and keep the temperature at 60°C±5°C for 30 minutes;
[0055] (4) Decarburization, after detecting the content and pH of the filtrate, add a phosphate buffer solution with a pH value of 2.0, filter the solution through a two-stage 0.22-micron microporous membrane, and then fill it in a container that has been sterilized at >350°C for >5 minutes ampoule.
[0056] (5) Sterilization, water bath sterilization at 115°C for 40 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com