Immediately-release haemophilus Type B conjugate vaccine soluble microneedle and preparation method thereof
A Haemophilus influenzae-conjugated vaccine technology is applied in the field of immediate-release Haemophilus influenzae type b conjugated vaccine soluble microneedle patch and its preparation, and can solve the problems of wasting antigen vaccines, poor immunization effect, and unsuitable inoculation times.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of Haemophilus influenzae type b conjugate vaccine soluble microneedle patch without adjuvant added
[0029] Prepared by the following method:
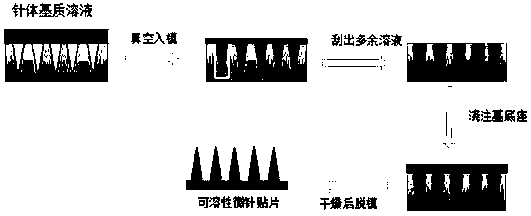
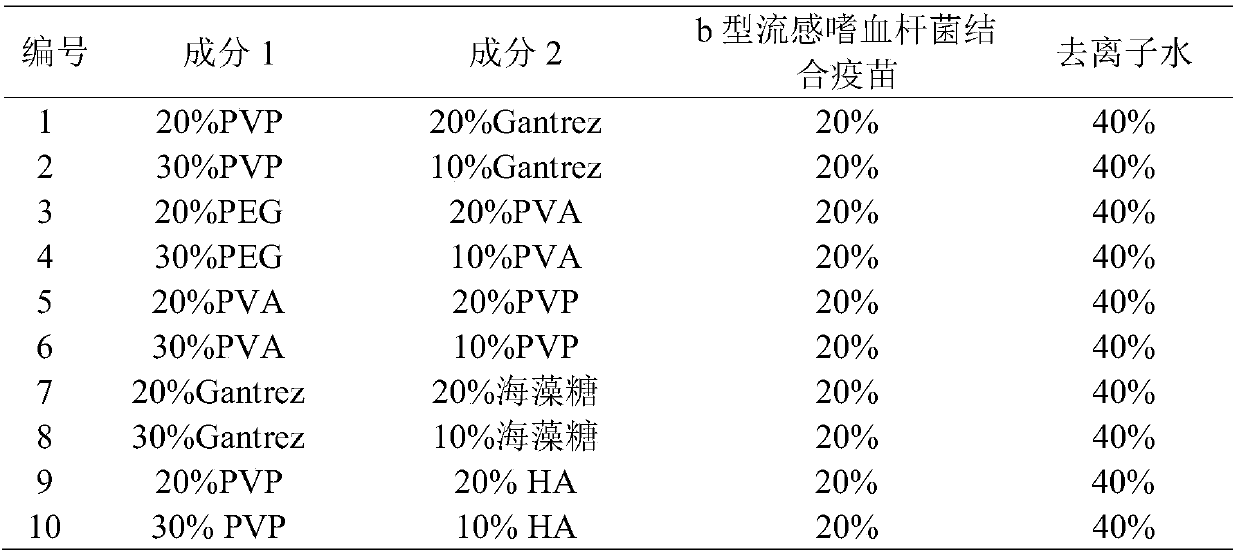
[0030] 1) Preparation of needle solution: dissolve the water-soluble polymer material (taking PVP and Gantrez as an example, based on a content ratio of 3:1, but not limited to this), Haemophilus influenzae type b conjugate vaccine, etc. in water , as a needle matrix solution.
[0031] 2) Preparation of the base solution: the flexible polymer material (take hypromellose as an example, but not limited thereto) is dissolved in water as the base solution.
[0032] 3) Preparation of soluble microneedles: inject the needle body solution into the microneedle female mold, centrifuge at 4000rpm for 20min, scrape off the excess needle body solution, put it in a normal temperature drying oven for 1h, then add the base layer solution, and centrifuge again at 4000rpm for 20min , put the negative mold of the microne...
Embodiment 2
[0036] Embodiment 2: Comparison of the dissolution performance of the soluble microneedle patch prepared in Example 1 in the skin
[0037] After the soluble microneedles of Haemophilus influenzae type b conjugate vaccine were prepared according to the different prescriptions of the above-mentioned Example 1, the microneedles were pressed into the skin of 6 different rats with the thumb, and the microneedles were pressed into the skin of 6 different rats at 0.2 min, 0.5 min, 1 min, and 2 min respectively. , 3min, 4min, 5min, 6min, 7min and other time points, the microneedles were pulled out, and the intradermal dissolution of the microneedles was observed through a microscope. The results are shown in Table 2.
[0038] Table 2 Intradermal dissolution of each microneedle (n=6)
[0039]
[0040] It can be seen from Table 2 that the dissolution rate of No. 2 prescription in the microneedle skin is better than other prescriptions, so the water-soluble material in the needle bod...
Embodiment 3
[0041] Example 3: Preparation of adjuvanted Haemophilus influenzae type b conjugate vaccine soluble microneedle patch
[0042] With reference to Examples 1 and 2, it is prepared by the following method:
[0043] 1) Preparation of the needle body solution: the water-soluble polymer material PVP and Gantrez (according to the content ratio of 3:1), Haemophilus influenzae type b conjugate vaccine, heat-resistant protective agent (taking trehalose as an example, But not limited thereto) and adjuvant (see the adjuvant prescription in Table 3) were dissolved in water as the needle matrix solution. In this embodiment, in order to eliminate unnecessary influencing factors, the contents of water-soluble polymer materials and heat-resistant protective agents are limited to fixed values for the convenience of statistics, but it is not limited thereto.
[0044] 2) Preparation of the base solution: the flexible polymer material (take hypromellose as an example, but not limited thereto) i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com