Method for preparing cross-linked alkaline anion exchange membrane based on flexible long side chain polycation structure
A basic anion and polycation technology, applied in the direction of climate sustainability, final product manufacturing, sustainable manufacturing/processing, etc., can solve problems such as low electrical conductivity and poor chemical stability, and achieve improved mechanical properties and ease mechanical strength Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Copolymerization into polymer backbone
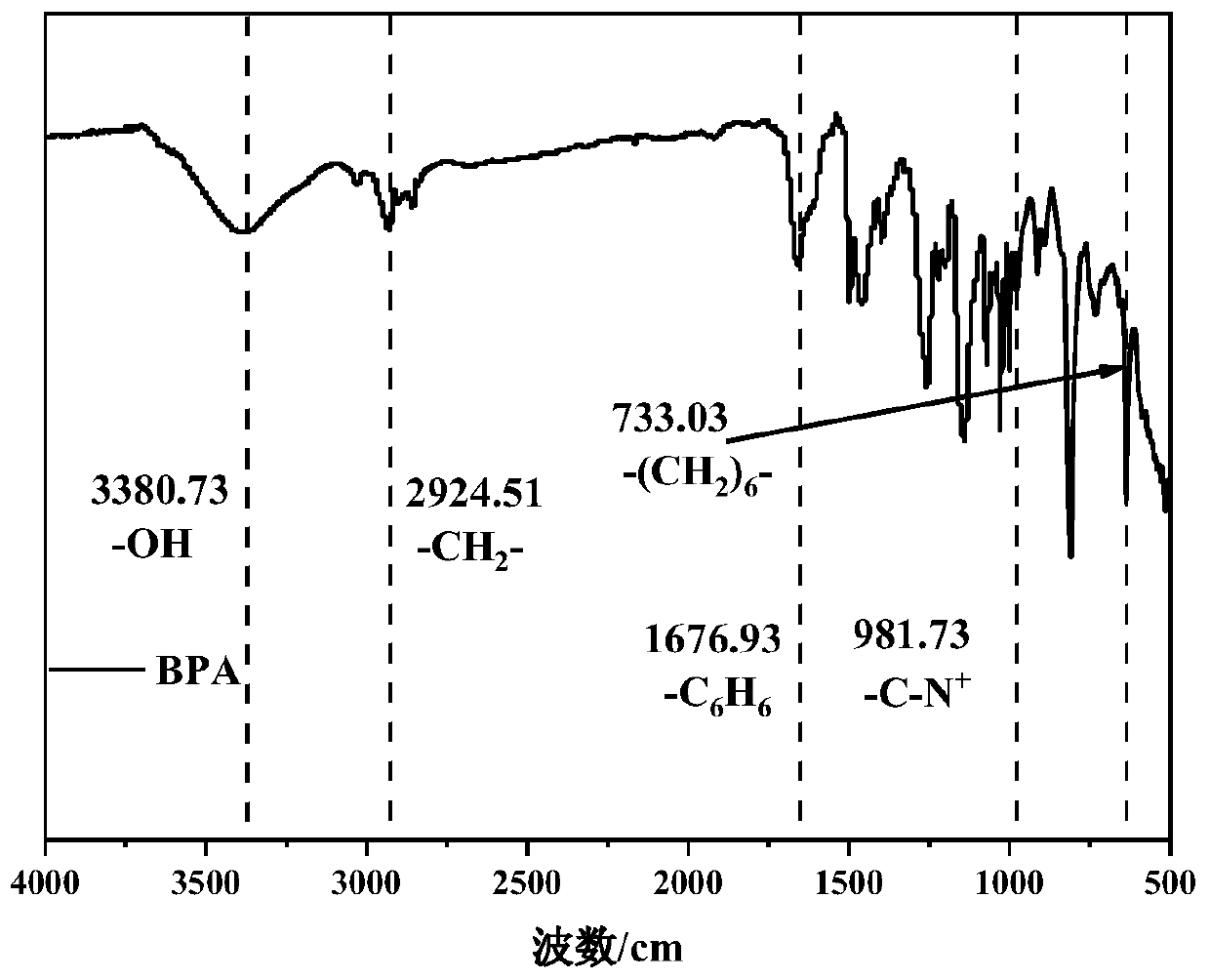
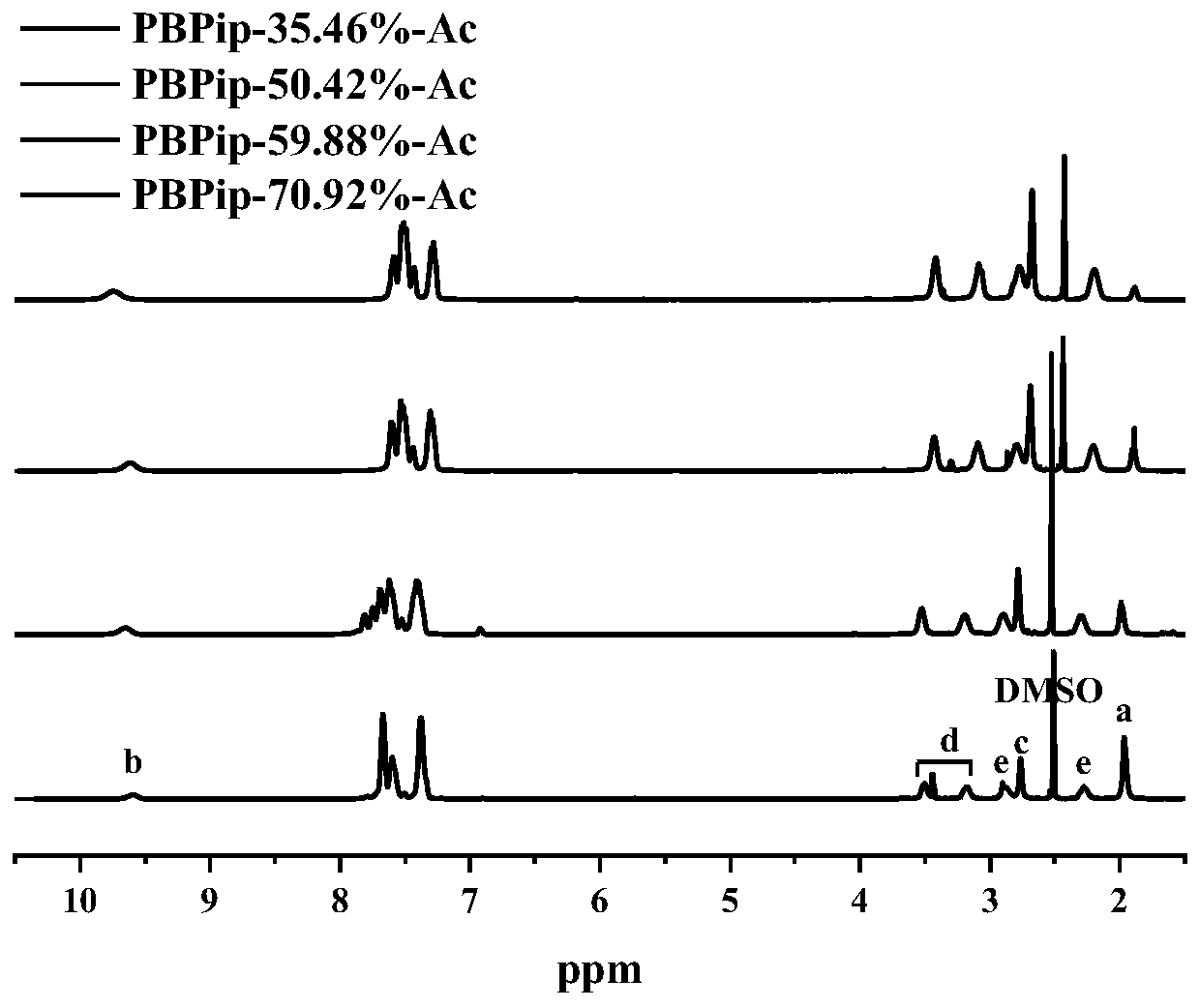
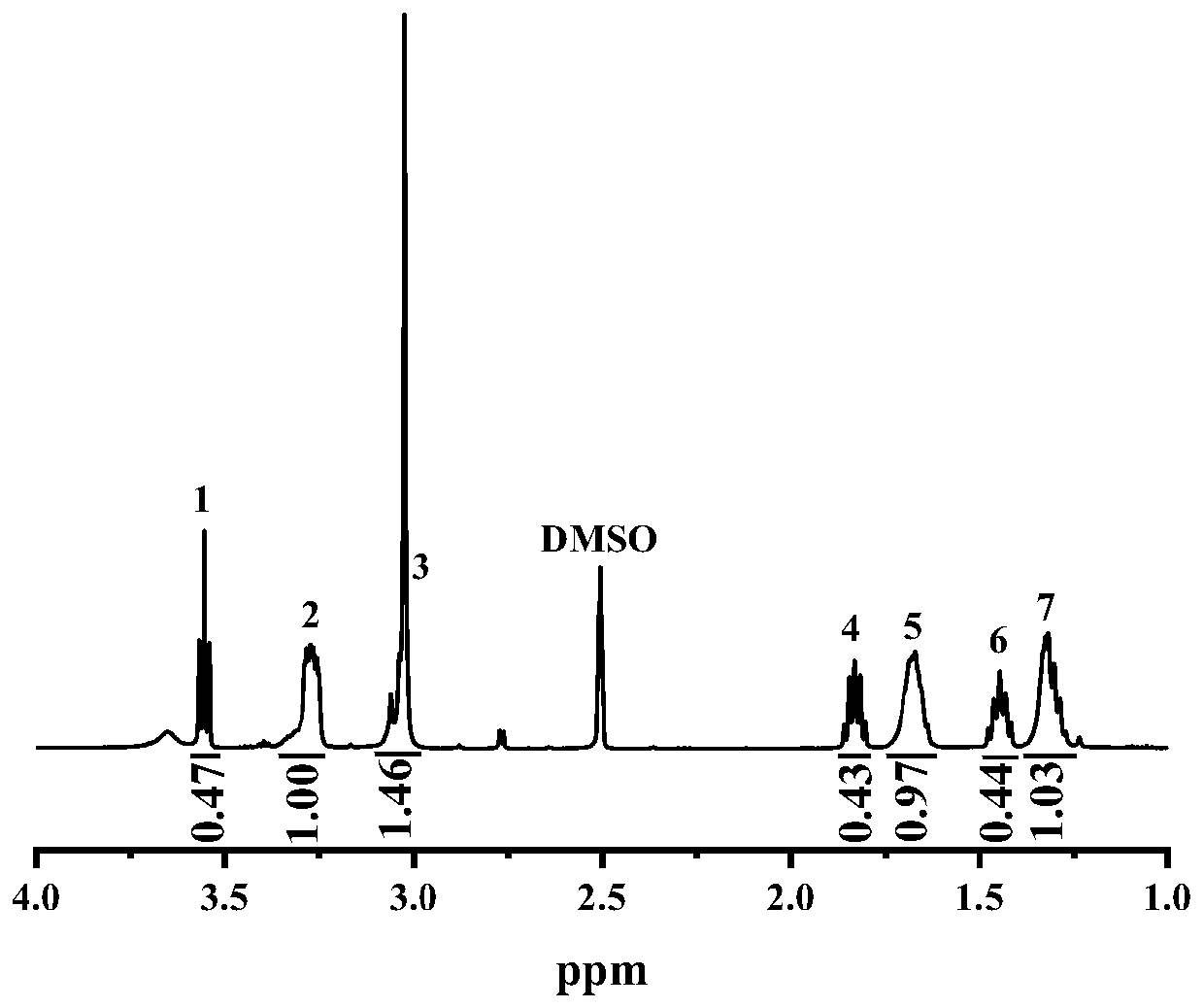
[0037] Dissolve 2.5g of biphenyl in 6mL of dichloromethane, and add a mixed reagent consisting of N-methylpiperidone and 1,1,1-trifluoroacetone in a molar ratio of 1:0 under the protection of nitrogen atmosphere, wherein N-methylpiperidone 1.2 mL. Then trifluoroacetic acid and trifluoromethanesulfonic acid were added as catalysts, the molar ratio of trifluoroacetic acid and trifluoromethanesulfonic acid was 1:6, and 1.8 mL of trifluoroacetic acid was added. The reaction was carried out under ice bath for 2h. The reaction mixture was poured into 1M NaOH precipitant for precipitation, washed with deionized water to neutrality, and vacuum-dried at 40°C for 48 hours to obtain a polymer: a copolymerized biphenyl-based oxygen-free main chain with a functionalization degree of 1 mmol g -1 .
[0038] (2) Preparation of ionization reagent
[0039] Take 2 mL of butanediamine and add it to ethanol to form a dropwise volume fraction ...
Embodiment 2
[0047] (1) Copolymerization into polymer backbone
[0048] Dissolve 2.5g of biphenyl in 6mL of dichloromethane, and add a mixed reagent consisting of N-methylpiperidone and 1,1,1-trifluoroacetone in a molar ratio of 1:1 under the protection of nitrogen atmosphere, wherein N-methylpiperidone 1.2 mL. Then trifluoroacetic acid and trifluoromethanesulfonic acid were added as catalysts, the molar ratio of trifluoroacetic acid and trifluoromethanesulfonic acid was 1:7, and 1.8 mL of trifluoroacetic acid was added. The reaction was carried out under ice bath for 3.5h. The reaction mixture was poured into 1M NaOH solution for precipitation, washed with deionized water until neutral, and vacuum-dried at 70°C for 30 hours to obtain a polymer: a copolymerized biphenyl-based oxygen-free main chain with a functionalization degree of 0.5 mmol g -1 .
[0049] (2) Preparation of ionization reagent
[0050] 2 mL of butanediamine was added to ethanol to form a co-solution with a volume fracti...
Embodiment 3
[0058] (1) Copolymerization into polymer backbone
[0059] Dissolve 2.5g of biphenyl in 6mL of dichloromethane, and add a mixed reagent consisting of N-methylpiperidone and 1,1,1-trifluoroacetone with a molar ratio of 1:3 under the protection of nitrogen atmosphere, wherein N-methylpiperidone 1.2 mL. Then trifluoroacetic acid and trifluoromethanesulfonic acid were added as catalysts, the molar ratio of trifluoroacetic acid and trifluoromethanesulfonic acid was 1:8, and 1.8 mL of trifluoroacetic acid was added. The reaction was carried out under ice bath for 5h. The reaction mixture was poured into 1M NaOH precipitant for precipitation, washed with deionized water to neutrality, and vacuum-dried at 100°C for 12 hours to obtain a polymer: a copolymerized biphenyl-based oxygen-free main chain with a functionalization degree of 0.3 mmol g -1 .
[0060] (2) Preparation of ionization reagent
[0061] 2 mL of butanediamine was added to ethanol to form a co-solution with a volume ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com