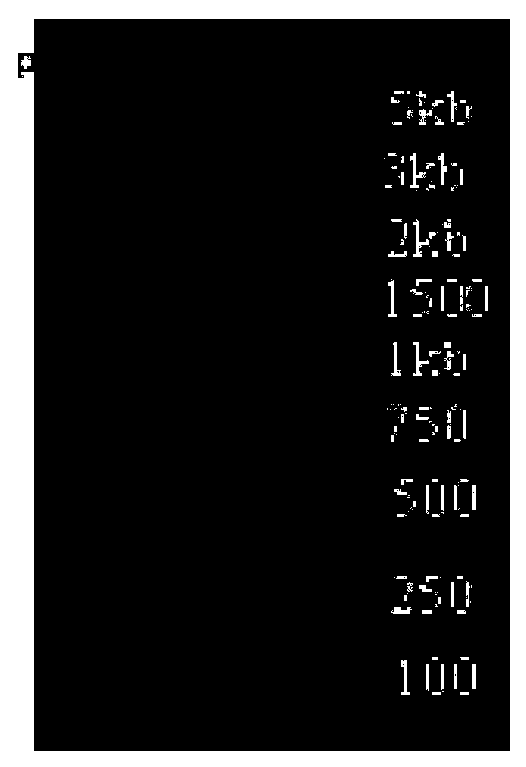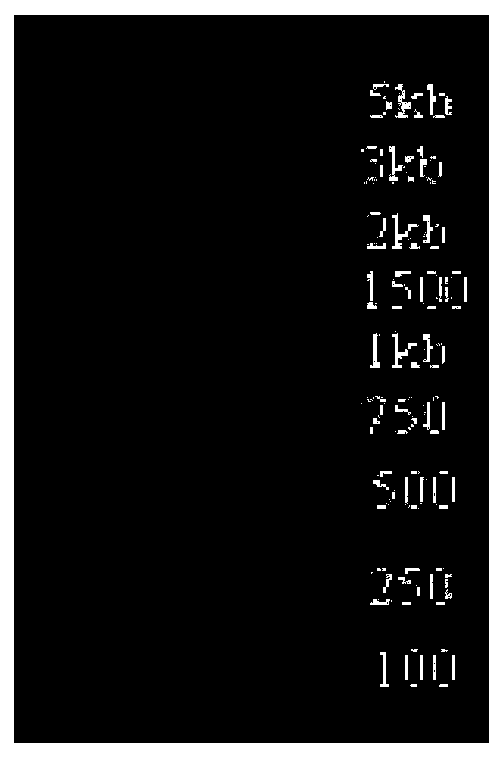Expression of recombinant human papilloma virus subtype 6 and 11 protein in pichia pastoris
A human papilloma and expression vector technology, applied in antiviral agents, viral antigen components, recombinant DNA technology, etc., can solve the problems of low HPV protein expression efficiency, unsatisfactory particle immune effect, low protein activity, etc. The effect of large-scale industrial production, low cost and high expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 HPV6 and 11 L1 codon optimization design
[0055] There are 64 genetic codes, but most organisms tend to use some of these codons. Pichia pastoris and human genes have their own preferences for codon usage. Since the genetic code is degenerate, each amino acid is encoded by more than one codon, and the codons of the same amino acid are used differently in wild-type genes. The codon preference of Pichia pastoris may lead to low translation efficiency and expression level of recombinant protein. The present inventors are concerned about the wild-type HPV 6 L1 gene (Genebank FR751337.1) and the wild-type HPV 11 L1 gene (Genebank HE611271.1). Carry out transformation: All its amino acids adopt the most frequently used codons. The frequency of Pichia yeast codon usage is shown in Table 1 (see http: / / www.kazusa.or.jp / codon / ). Then, on this basis, and in order to avoid the excessively high GC ratio of the translated mRNA, the secondary structure of the mRNA affects t...
Embodiment 2
[0062] Example 2 Construction of HPV6 and 11 L1 recombinant expression vectors
[0063] The synthesized HPV6 L1 sequence of SEQ ID NO: 2 was cloned into the pPICZalphaB vector (Invitrogen) by the following method.
[0064] 6 L1 DNA fragments with BstBI and KpnI on both ends were amplified by PCR. PCR program: 94°C for 5 minutes, 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute and 50 seconds, cycle 30 times, 72°C for 10 minutes, 10°C for 10 minutes, and the operation ends. The PCR products were identified by agarose gel electrophoresis and a 1500bp band was recovered (Qiagen gel extraction kit). The recovered fragments and pPICZalphaB were digested with BstBI and KpnI (New England Biolab), identified by agarose gel electrophoresis, and 1500bp and 3600bp fragments were recovered respectively. After recovery, 6L1 and pPICZalphaB were ligated with T4 ligase (Takara) overnight at 16°C, and the ligation product was transformed into E.coli DH5α the next day, spread on an LB ...
Embodiment 3
[0067] Example 3 Construction and expression of HPV6 and 11 L1 recombinant expression strains
[0068] Linearize pPICZ6L1 with SacI. After the enzyme digestion reaction, remove the protein from phenol: chloroform, then add 2.5 times volume of absolute ethanol, 1 / 10 volume of 3M NaAc (pH 5.2) to precipitate DNA, and the resulting precipitate is washed with 75% ethanol and dried After a small amount of sterile ddH 2 O dissolves the precipitate, electrotransforms the Pichia X-33 strain (Invitrogen), spreads it on a YPDS plate (containing 200μg / ml Zeocin), cultivates at 30°C for 3 days to obtain hundreds of clones. Pick dozens of clones and inoculate them on YPD plates (containing 1500μg / ml Zeocin), select high-copy plasmid strains, and culture them at 30°C for 2 days. Some clones grow faster. Pick the best clones and inoculate them in 4ml YPD liquid medium. Replace the BMMY medium 24 hours later, and collect the bacteria after 48 hours of induction with 0.5% methanol. After the bac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com