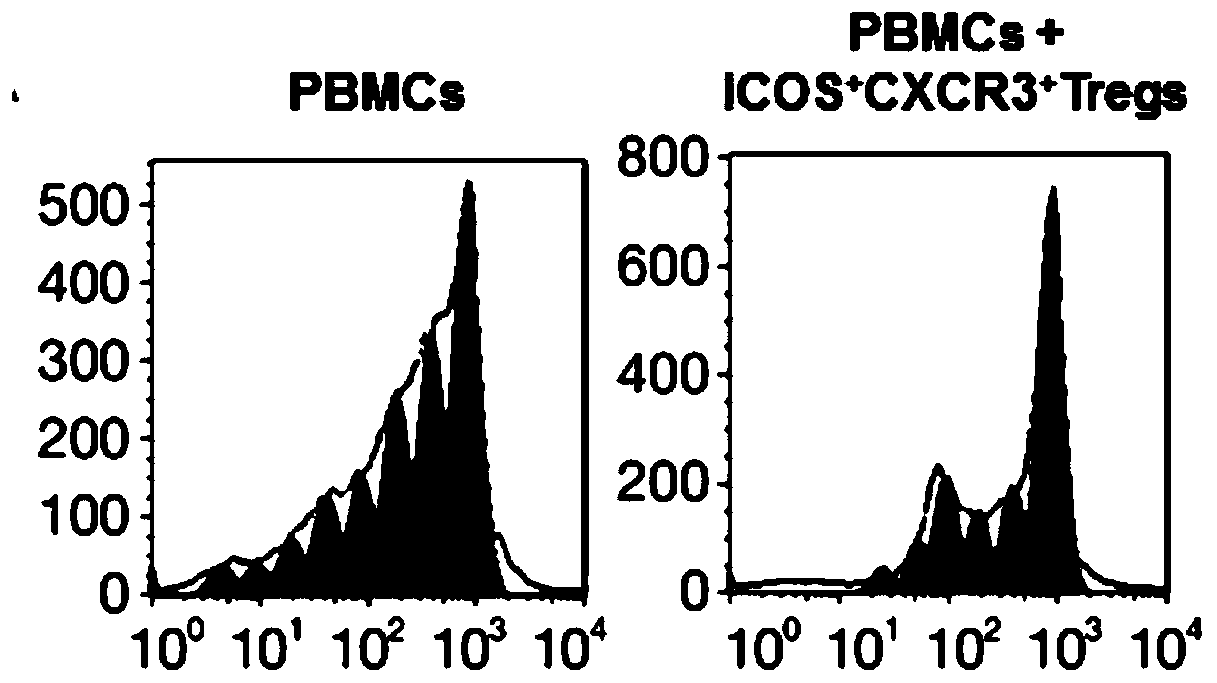
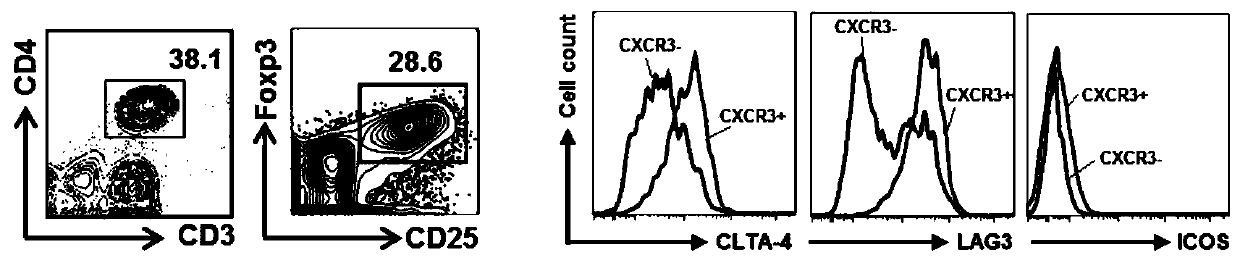
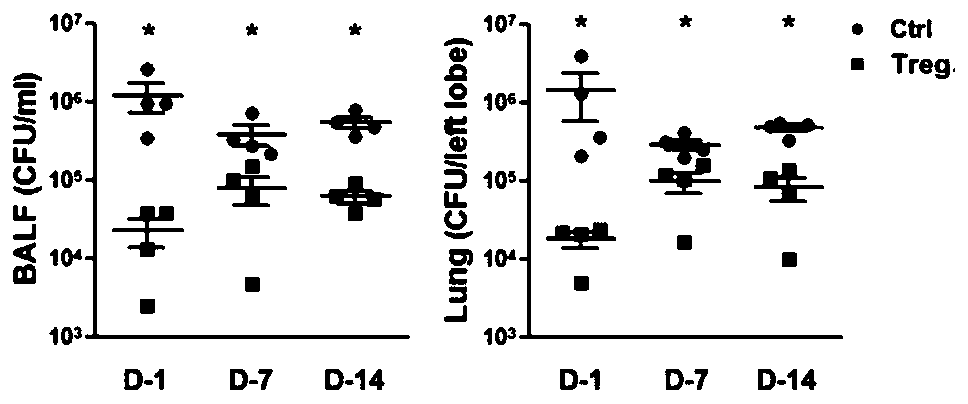
Application of ICOS<+>CXCR3<+> regulatory T cells in preparation of medicine for preventing severe pneumonia
A technology for severe pneumonia and regulation, applied in the field of biomedicine, can solve the problems such as disputes in the adjuvant treatment of severe pneumonia, and achieve the effect of good prevention and protection, high degree of technology promotion, and avoidance of side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] ICOS + CXCR3 + In vitro isolation and expansion of regulatory T cells
[0026] 1. ICOS + CXCR3 + Isolation of regulatory T cells
[0027] Step 1: Separating mouse spleen mononuclear cells to obtain mouse spleen mononuclear cell suspension. The specific process is:
[0028] 1) Preparation of materials and reagents: mouse dissecting instruments, 75% alcohol, sterile PBS buffer, complete mouse spleen and lung cell culture medium (RPMI1640 medium with 10% calf serum), erythrocyte lysate, 200 mesh nylon membrane.
[0029] 2) Mice were killed by dislocation of the cervical spine, soaked in 75% alcohol for 10 minutes for disinfection, taken out and fixed on the mouse dissecting board.
[0030] 3) Use ophthalmic scissors to cut from the perineum of the mouse and draw it to the head, peel off the outer skin and fix it, cut the incision at the abdominal capsule, take out the spleen with ophthalmic scissors and tweezers, add an appropriate amount of pre-cooled PBS buffer, a...
Embodiment 2
[0055] ICOS + CXCR3 + Administration of regulatory T cells
[0056] 1. Final identification before use
[0057] 1. Sterility test
[0058] If there is no abnormality during the culture process, the ICOS + CXCR3 + Two days before the regulatory T cells were infused into the mice, after the last medium change, a sterility test was performed on the supernatant in each culture dish, and bacteria and mold were cultured. The cell supernatant must not have bacteria and mold growth.
[0059] 2. Appearance and observation under the microscope
[0060] On the day of infusion, if the sterility test reports no growth of bacteria or mold, observe the color of the supernatant in the petri dish, observe the cell shape, cell density, and whether there is growth of bacteria or mold under an inverted microscope. If there is any doubt, another sample must be taken for sterility test to cultivate bacteria and mold.
[0061] 3. Proportion of living cells
[0062] On the day of infusion, sam...
Embodiment 3
[0079] ICOS + CXCR3 + Application of regulatory T cells in the preparation of drugs for preventing severe bacterial pneumonia animal models
[0080] 1. Preparation of bacteria solution for Haemophilus influenzae infection
[0081] 1) Take out the Haemophilus influenzae NT127 frozen at -80°C, dip a little with a sterilized wooden stick, inoculate it on the sBHI culture plate by streaking, and cultivate overnight in a carbon dioxide incubator at 37°C.
[0082] 2) Pick multiple single colonies and inoculate them in 10 ml of sBHI liquid medium, culture on a shaker at 37° C. at 130 rpm until OD600 = 0.4-0.5.
[0083] 3), room temperature, 4000rpm, centrifuge for 15 minutes, collect bacteria.
[0084] 4) Bacteria were resuspended in 12ml of PBS buffer, centrifuged at 4000rpm at room temperature for 15 minutes, and the bacteria were collected. Resuspend in PBS buffer, adjust OD600=1.0, at this time the bacterial concentration is about 3×109CFU / ml.
[0085] 5), 10-fold gradient d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com