Preparation method of dialkyl sulfone compounds
A technology of dialkyl sulfone and salt compounds, which is applied in the field of preparation of dialkyl sulfone compounds, can solve problems such as difficulty in applying target products, and achieve good guiding significance and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
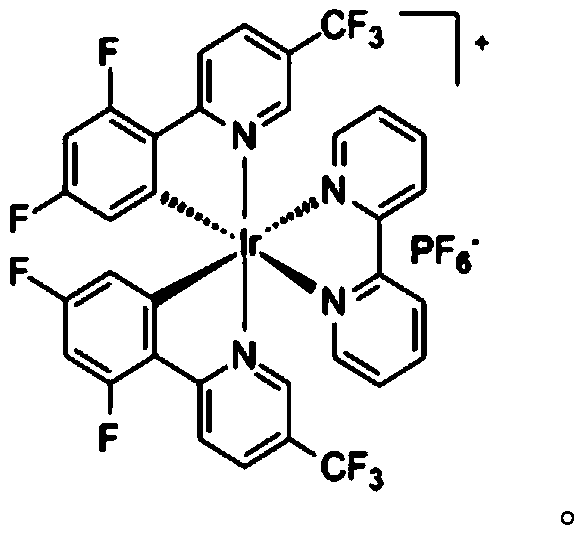
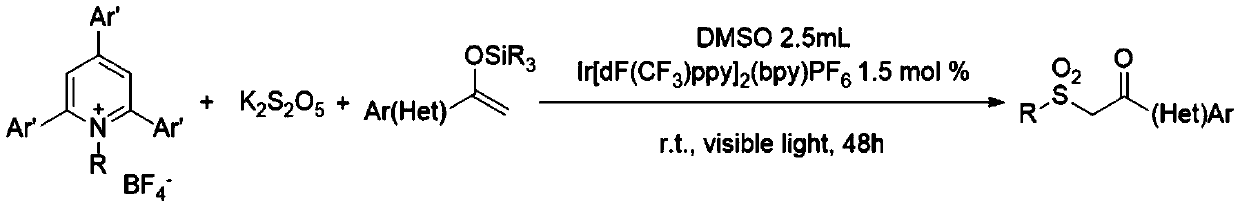
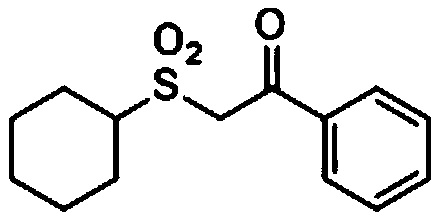
[0031] Add 0.2mmol of N-cyclohexyl-2,4,6-triphenylpyridine tetrafluoroborate, 0.4mmol of potassium metabisulfite, 0.003mmol (1.5mol%) of iridium photosensitizer Ir[ dF(CF 3 )ppy] 2 (bpy)PF 6, stop the reaction tube with a stopper and place it in high-purity nitrogen to replace the gas, so that the system is in anhydrous and oxygen-free conditions, and then add 2.5mL of dry dimethyl sulfoxide and 0.6mmol of 1-phenyl-1-triisopropyl Siloxyethylene, placed in a visible light reaction device and stirred until completely reacted. After the reaction was monitored by TCL, pour the reaction liquid into 50 mL of water, extract three times with 20 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, concentrate under reduced pressure, and use a mixture of petroleum ether and ethyl acetate as the mobile phase The corresponding 2-(cyclohexylsulfonyl)-1-phenyl-1-ethanone (2-(Cyclohexylsulfonyl)-1-phenylethan-1-one) example 1 can be obtained by c...
Embodiment 2
[0034]
[0035] Add 0.2mmol of N-cyclohexyl-2,4,6-triphenylpyridine tetrafluoroborate, 0.25mmol of potassium metabisulfite, 0.002mmol (1.5mol%) of iridium photosensitizer Ir[ dF(CF 3 )ppy] 2 (bpy)PF 6 , stop the reaction tube with a stopper and place it in high-purity argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 2.5 mL of dry dimethyl sulfoxide and 0.4 mmol of 1-(4-methylphenyl) -1-Triisopropylsiloxyethylene, placed in a visible light reaction device and stirred until complete reaction. After the reaction was monitored by TCL, the reaction liquid was poured into 50 mL of water, extracted three times with 20 mL of ether, the combined organic phases were dried with anhydrous sodium sulfate, concentrated under reduced pressure, and a mixture of petroleum ether and ethyl acetate was used as the mobile phase for column extraction. Chromatographic separation, the corresponding 2-(cyclohexylsulfonyl)-1-(4-methylphenyl)-1-etha...
Embodiment 3
[0038]
[0039] Add 0.2 mmol of N-cyclohexyl-2,4,6-triphenylpyridine tetrafluoroborate, 0.45 mmol of potassium metabisulfite, 0.004 mmol (1.5 mol%) of iridium photosensitizer Ir to the dry test tube[ dF(CF 3 )ppy] 2 (bpy)PF 6 , stop the reaction tube with a stopper and place it in high-purity nitrogen to replace the gas, so that the system is in anhydrous and oxygen-free conditions, and then add 2.5 mL of dry dimethyl sulfoxide and 0.7 mmol of 1-(4-trifluoromethylphenyl )-1-triisopropylsiloxyethylene, placed in a visible light reaction device and stirred until complete reaction. After the reaction was monitored by TCL, pour the reaction liquid into 50 mL of water, extract three times with 20 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, concentrate under reduced pressure, and use a mixture of petroleum ether and ethyl acetate as the mobile phase Carry out column chromatography separation, can obtain corresponding 2-(cyclohexylsulfony...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com