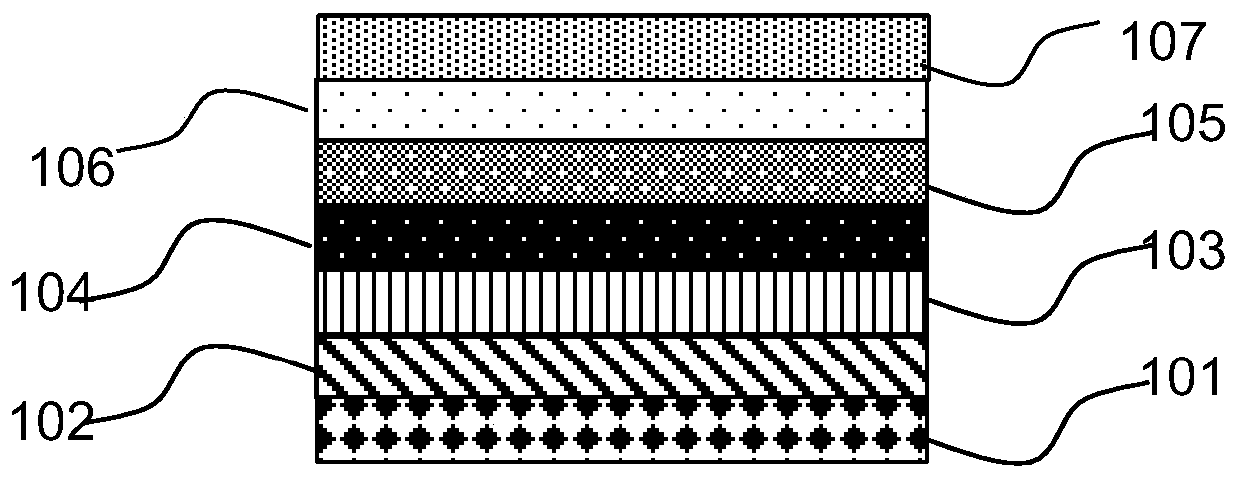
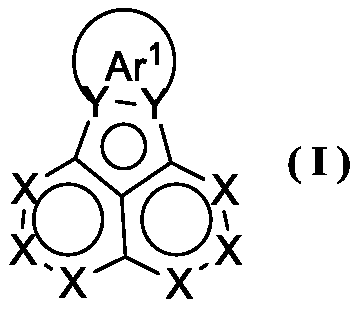
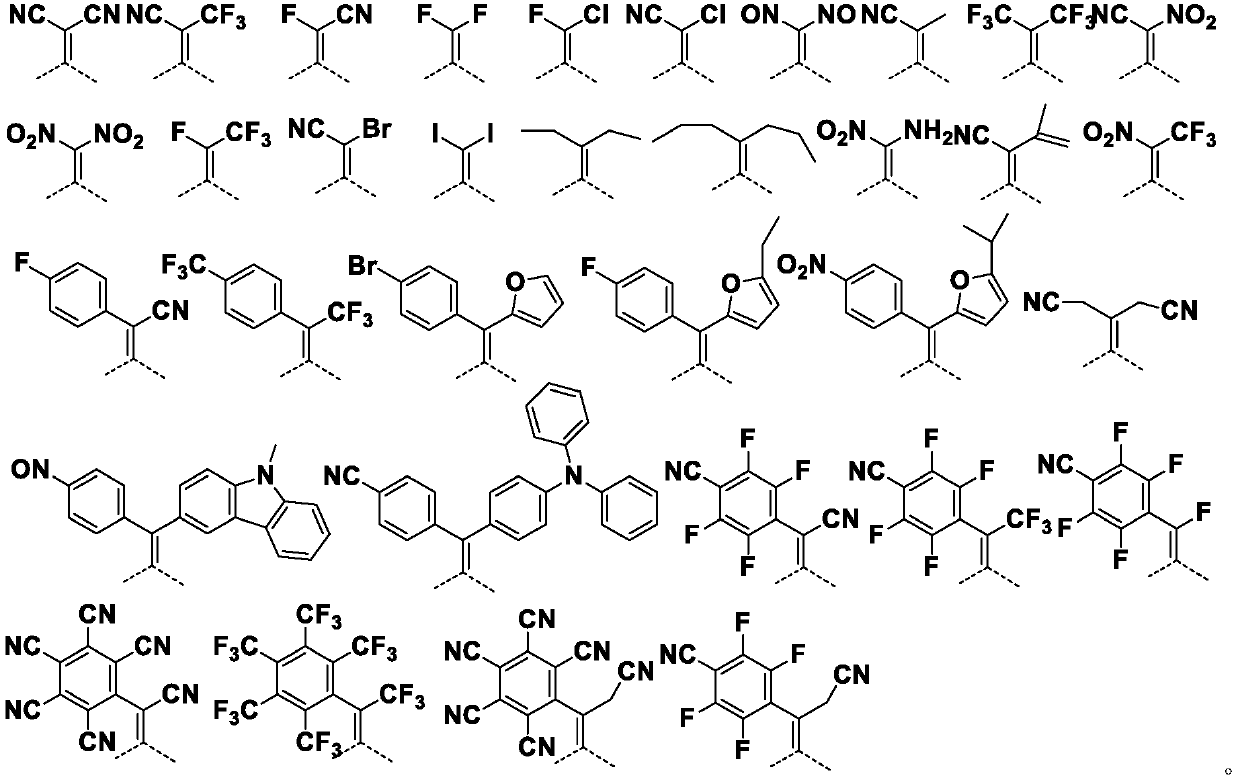
Acenaphthene quinone organic compound and application thereof
An organic compound, acenaphthylene quinone technology, applied in the field of organic electroluminescence, can solve the problems of insufficient stability, reduced lifespan of organic light-emitting diodes, decreased lifespan, etc., achieve excellent hole transport properties and stability, and improve electroluminescence efficiency. , the effect of prolonging the life of the device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Embodiment 1 synthetic compound DPD-1
[0199]
[0200] Synthesis of compound A2:
[0201] Compound A1 (1.82g, 10mmol) was dissolved in 80ml DMF, 7.4g (41.6mmol) NBS was dissolved in 73ml DMF solvent, and then the NBS solution was added dropwise into the substrate solution at a rate of 3-5 drops per second, and stirred. At normal temperature, the reaction stopped after the dropwise addition, and 30ml of water was added dropwise to the reaction solution, recrystallized, and suction filtered to obtain product A2 (2.01g, 60%) MS: [M+H] + =338.
[0202]
[0203] Synthesis of compound A3:
[0204] Sodium tert-butoxide (2.43g, 25mmol) and malononitrile (1.32g, 20mmol) were stirred for 15min under nitrogen and dry tetrahydrofuran, and A2 (3.37g, 10mmol), Pd(PPh 3 ) 4 (3.4g, 3mmol), and cuprous iodide (3.9g, 20mmol) were then stirred at 60°C for 12 hours, then quenched with cold concentrated hydrochloric acid, concentrated in dichloromethane, dried with anhydrous so...
Embodiment 2
[0210] Embodiment 2 synthetic compound DPD-2
[0211]
[0212] Synthesis of compound DPD-2:
[0213] Compound A4 (3.11g, 10mmol), A6 (3.88g, 20mmol) and 10ml of acetic acid were heated overnight, the reaction mixture was cooled to room temperature, and the resulting solid was filtered and washed with ethanol and water to obtain DPD-2 (3.75g, 80%).
Embodiment 3
[0214] Embodiment 3 synthetic compound DPD-3
[0215]
[0216] Synthesis of compound DPD-3:
[0217] Compound A4 (3.11g, 10mmol), A7 (3.16g, 20mmol) and 10ml of acetic acid were heated overnight, the reaction mixture was cooled to room temperature, and the resulting solid was filtered and washed with ethanol and water to obtain DPD-3 (2.87g, 65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com