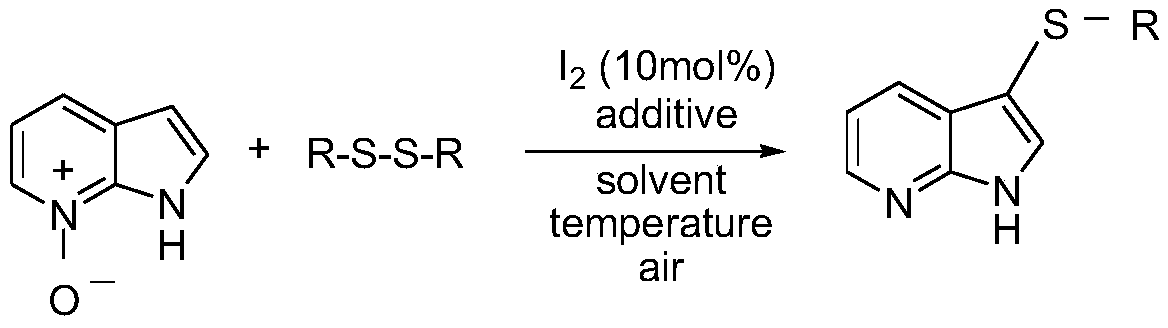
Regioselective deoxidation thionation reaction for 7-azaindole-nitrogen oxide
A regioselective, azaindole technology, applied in organic chemistry and other fields, can solve problems such as low efficiency, poor adaptability, high toxicity, etc., achieve great application prospects, increase universality, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
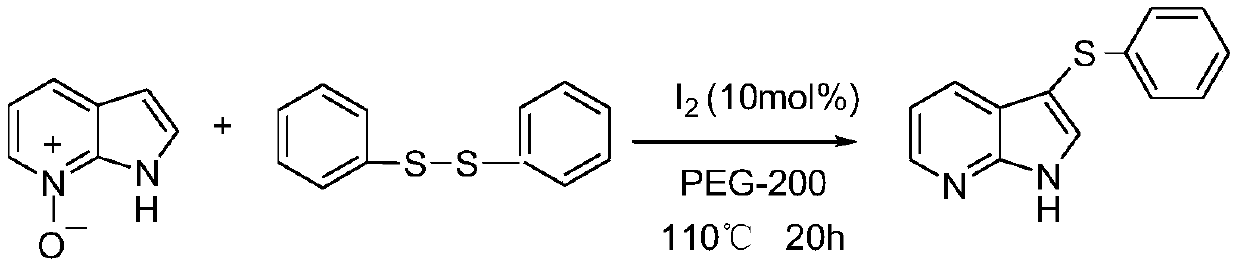
[0030] Under room temperature conditions, 53.6 mg (0.4 mmol) of 7-azaindole-nitrogen oxide, 52.3 mg (0.24 mmol) of diphenyl disulfide, molecular iodine (10 mol%) and PEG-200 ( 2.0mL); The mass ratio of 7-azaindole-nitrogen oxide and diphenyl disulfide is 1:0.6; the mass ratio of 7-azaindole-nitrogen oxide and iodine catalyst is 1:1; Add the measured reactants to a 25mL pressure tube equipped with magnets in turn, close the pressure tube, stir on a magnetic stirrer at room temperature, then slowly heat to 110°C, at 0.1MPa, The reaction was stopped after 20 hours, cooled to room temperature, the mixture was extracted with diethyl ether (10mL×3), the organic phases were combined, and the excess solvent was removed by a rotary evaporator, and the residue was separated by column chromatography, and 300-400 mesh silica gel As a stationary phase, the product is purified and separated with a mixed solvent of ethyl acetate and petroleum ether as an eluent.
[0031] The phy...
Embodiment 2
[0036]
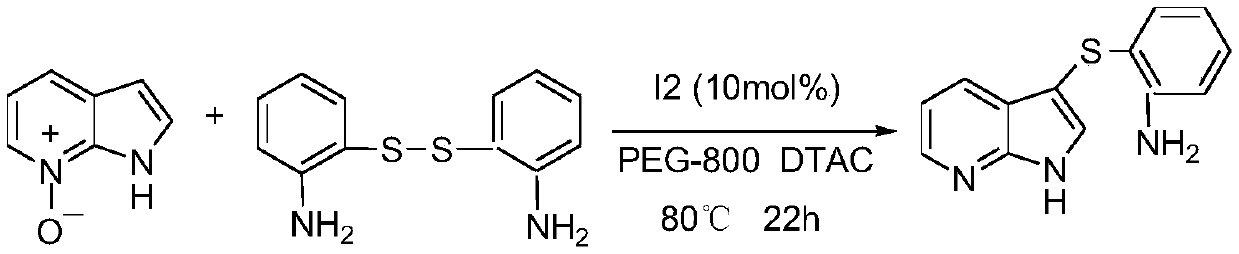
[0037] At room temperature, accurately weigh 53.6 mg (0.4 mmol) of 7-azaindole-nitrogen oxide, 99.2 mg (0.24 mmol) of bis(2-aminophenyl) disulfide, molecular iodine (10 mol%) and PEG-800 3mL; the molar ratio of 7-azaindole-nitrogen oxide to bis(2-aminophenyl) disulfide is 1:1; the ratio of 7-azaindole-nitrogen oxide to iodine catalyst The molar ratio of the substances is 1:0.5; the measured reactants are sequentially added to a 25mL pressure-resistant tube equipped with a magnet, and dodecyltrimethylammonium chloride (DTAC) is added, the pressure-resistant tube is closed, and the pressure-resistant tube is kept at room temperature Stir on a magnetic stirrer, then slowly heat to 80 ° C, under 0.1 MPa, stop the reaction after 22 h, cool to room temperature, extract the mixture with diethyl ether (10 mL × 3), combine the organic phases, and purify (same as in Example 1).
[0038] The physical properties and characterization data of the obtained compound are as follow...
Embodiment 3
[0043]
[0044] At room temperature, accurately weigh 53.6mg (0.4mmol) of 7-azaindole-nitrogen oxide, 137.76mg (0.48mmol) of 4,4'-dichlorodiphenyl disulfide, and molecular iodine (10mol% ) and toluene 5mL; 7-azaindole-nitrogen oxide and 4,4'-dichlorodiphenyl disulfide substance ratio is 1:1.2; 7-azaindole-nitrogen oxide and iodine The mass ratio of the catalyst is 1:0.8; the measured reactants are sequentially added to a 25mL pressure tube equipped with a magnet, and tetrabutylammonium bromide (TBAB) is added, the pressure tube is closed, and the Stir on a magnetic stirrer, then slowly heat to 100°C, under 0.1MPa, stop the reaction after 15h, cool to room temperature, extract the mixture with diethyl ether (10mL×3), combine the organic phases, and purify (same as
[0045] Example 1).
[0046] The physical properties and characterization data of the obtained compound are as follows:
[0047] white solid: 1 H NMR (400MHz, DMSO-d 6 ):δ=12.33(s,1H),8.32(dd,J=4.7Hz,J=1.6Hz,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com