Method for preparing fluorine-containing ethylene by catalytic cracking of 2-chloro-1,1-difluoroethane
A technology of difluoroethylene and difluoroethane, which is applied in the field of preparing fluorine-containing ethylene by catalytic cracking of 2-chloro-1,1-difluoroethane, can solve the problem of high reaction temperature, no industrial application value, high conversion rate and poor performance. Selectivity can not meet the requirements and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of 1,1-difluoroethylene of the present invention comprises the steps:
[0019] (i) A catalytic cracking catalyst is provided, the catalyst includes fluoride carried on activated carbon, the weight ratio of the activated carbon to the fluoride is 1:0.01~0.5, and the fluoride is selected from aluminum fluoride, chromium fluoride , potassium fluoride, magnesium fluoride, calcium fluoride, or a mixture of two or more thereof;
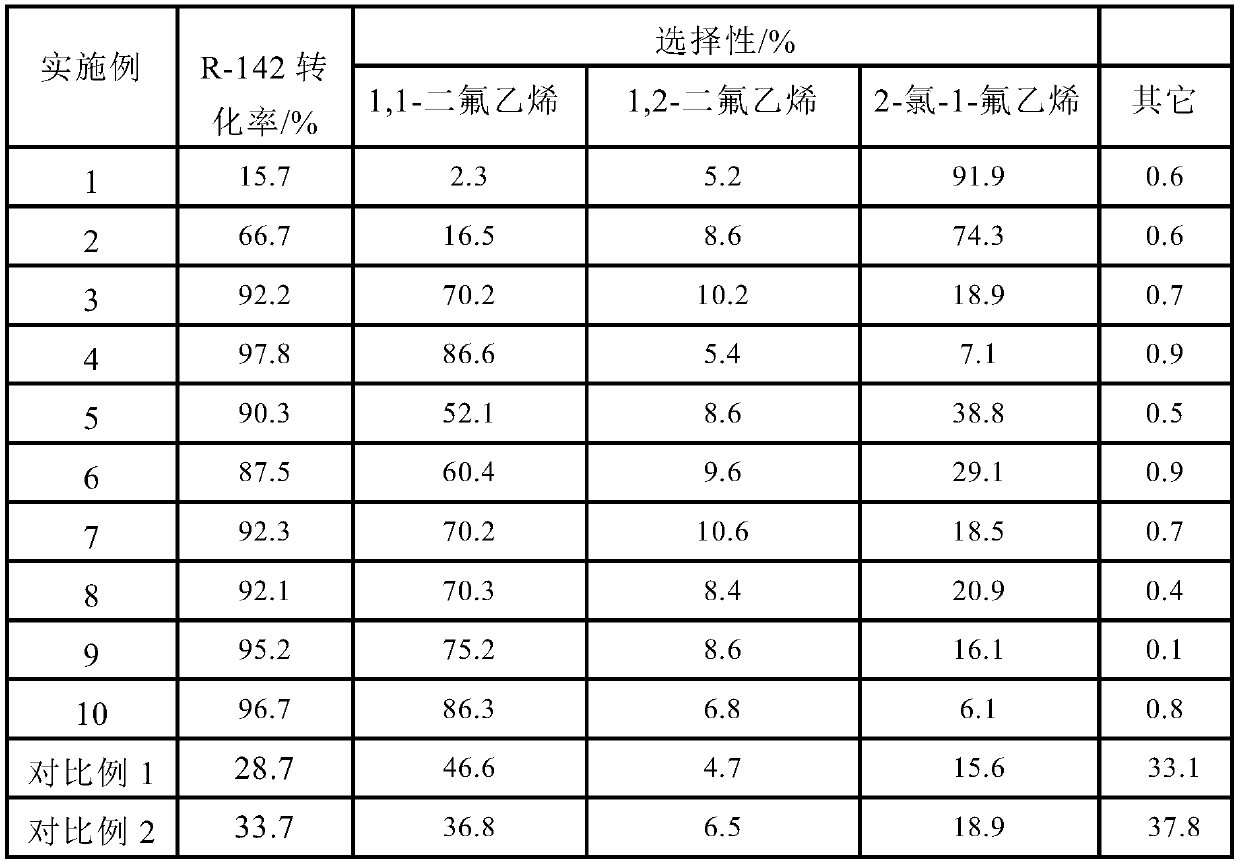
[0020] The inventors of the present invention tried to use the ferric fluoride supported on activated carbon mentioned in the prior art as a catalyst (see CN109180420A) to catalytically crack 2-chloro-1,1-difluoroethane, but found that although the catalyst was used for cracking The conversion rate of R-142b is as high as 93% and the ratio of VDF / chlorofluoroethylene in the product is as high as 99:1, but when it is used for catalytic cracking of R-142, the conversion rate is lower than 40%, and the ratio of VDF / chlorofluoroeth...
Embodiment 1
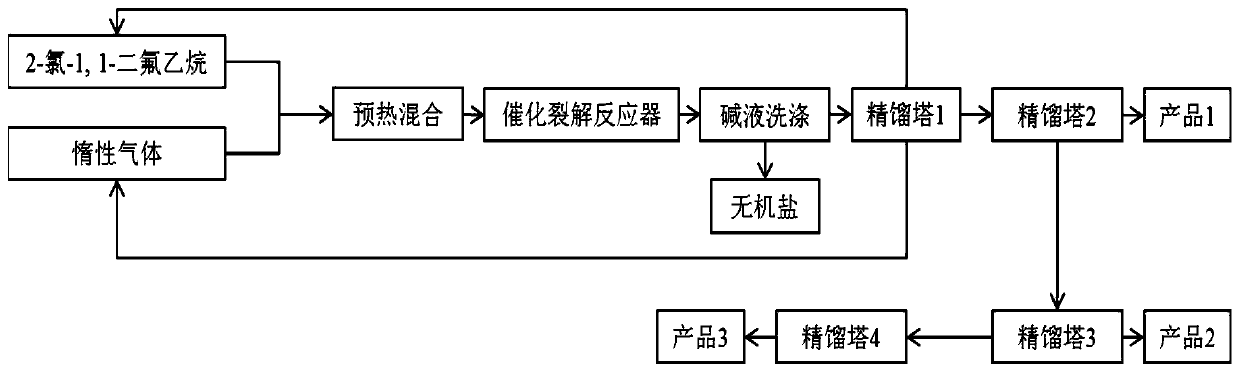
[0055] 100ml of activated carbon-supported aluminum fluoride catalyst particles (the weight ratio of activated carbon to aluminum fluoride is 1:0.3) was loaded into a 10-liter reactor bed to form a catalytic cracking reactor. Prepare 2-chloro-1,1-difluoroethane and nitrogen gas at a molar ratio of 2-chloro-1,1-difluoroethane to nitrogen as 1:10 to form a mixed gas, which is preheated at 250°C After passing into the catalytic cracking reactor, the catalyst was activated at 260°C for 1.5 hours, and then at 400°C, normal pressure, 1000h -1 The reaction is carried out at a space velocity of .
[0056] The reacted gas is washed by the KOH lye absorption tower to remove HF and HCl gas, and then the nitrogen and 2-chloro-1,1-difluoroethane separated by the mixed gas rectification tower 1 are recycled, containing fluorine The ethylene product gas is rectified through the rectification tower 2 to obtain 1,1-difluoroethylene gas with a purity of more than 99.9%. The separated tail gas...
Embodiment 2
[0059] 100ml of activated carbon-supported aluminum fluoride catalyst particles (the weight ratio of activated carbon to aluminum fluoride is 1:0.3) was loaded into a 10-liter reactor bed to form a catalytic cracking reactor. The molar ratio of 2-chloro-1,1-difluoroethane to argon is 1:10 to prepare 2-chloro-1,1-difluoroethane and argon gas to form a mixed gas, and the mixed gas is heated at 250°C After preheating, pass it into the catalytic cracking reactor, activate the catalyst at 260°C for 1.5 hours, and then at 550°C, normal pressure, 1000h -1 Reaction is carried out under the space velocity of; Reaction aftertreatment is with embodiment 1, and the tail gas of catalytic cracking reactor is carried out gas chromatographic analysis in reaction process, analysis result is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com