Succinylcholine chloride preparation method
A technology of succinylcholine chloride and choline chloride is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., and can solve problems such as inflammable, explosive, highly toxic, and inconvenient to handle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
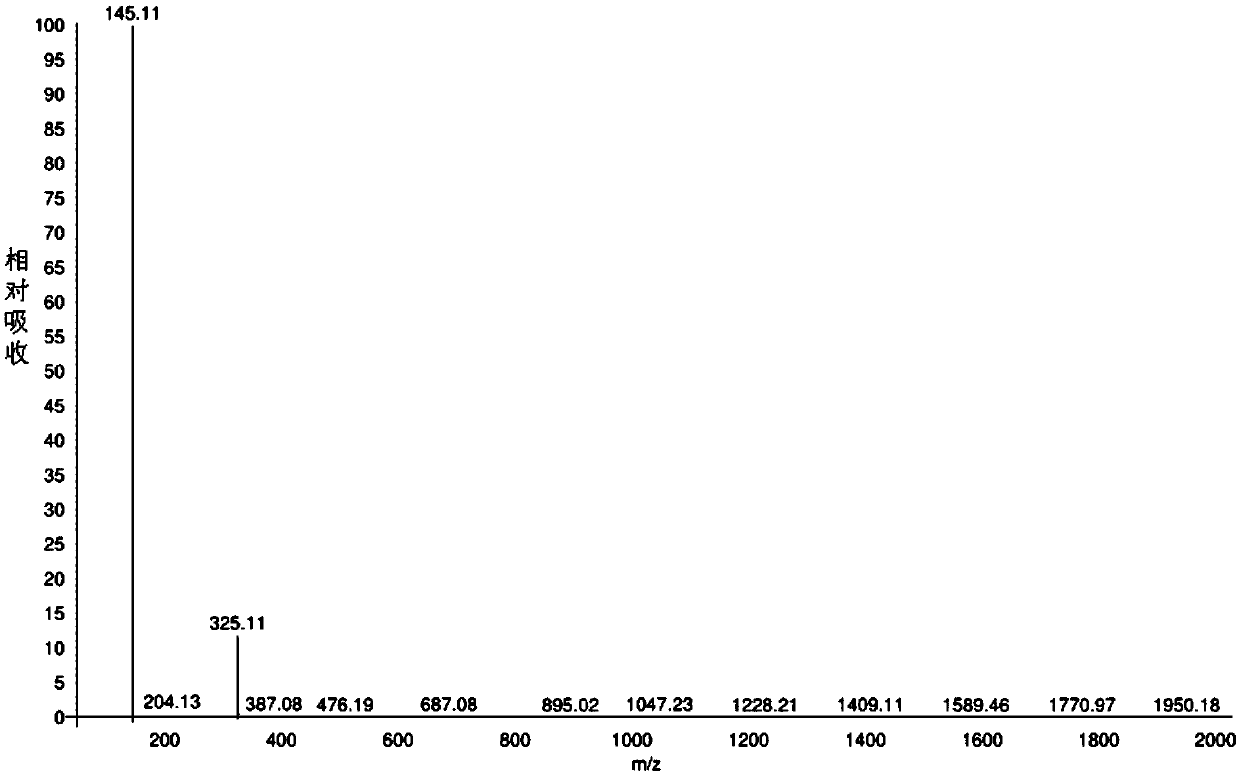
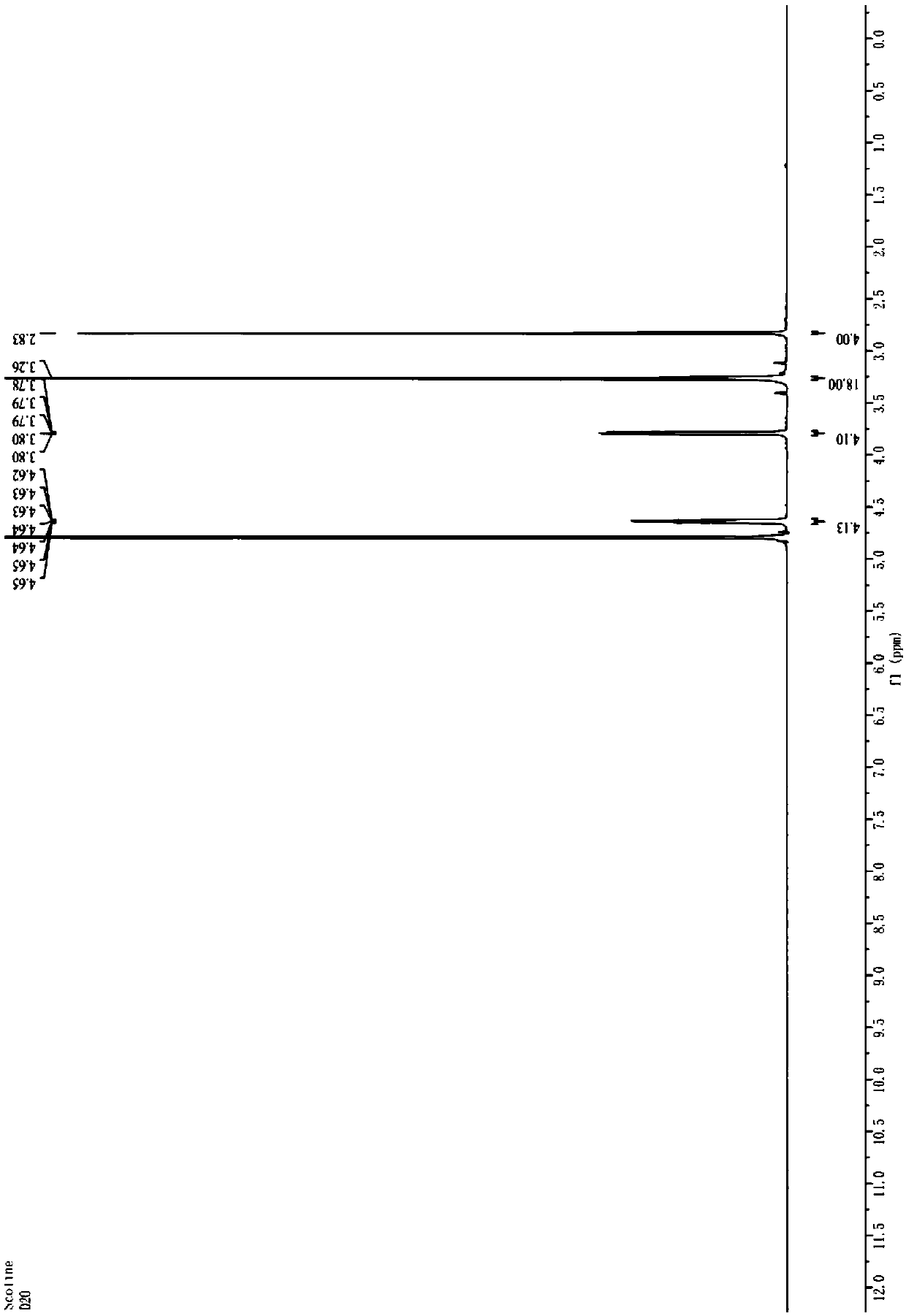
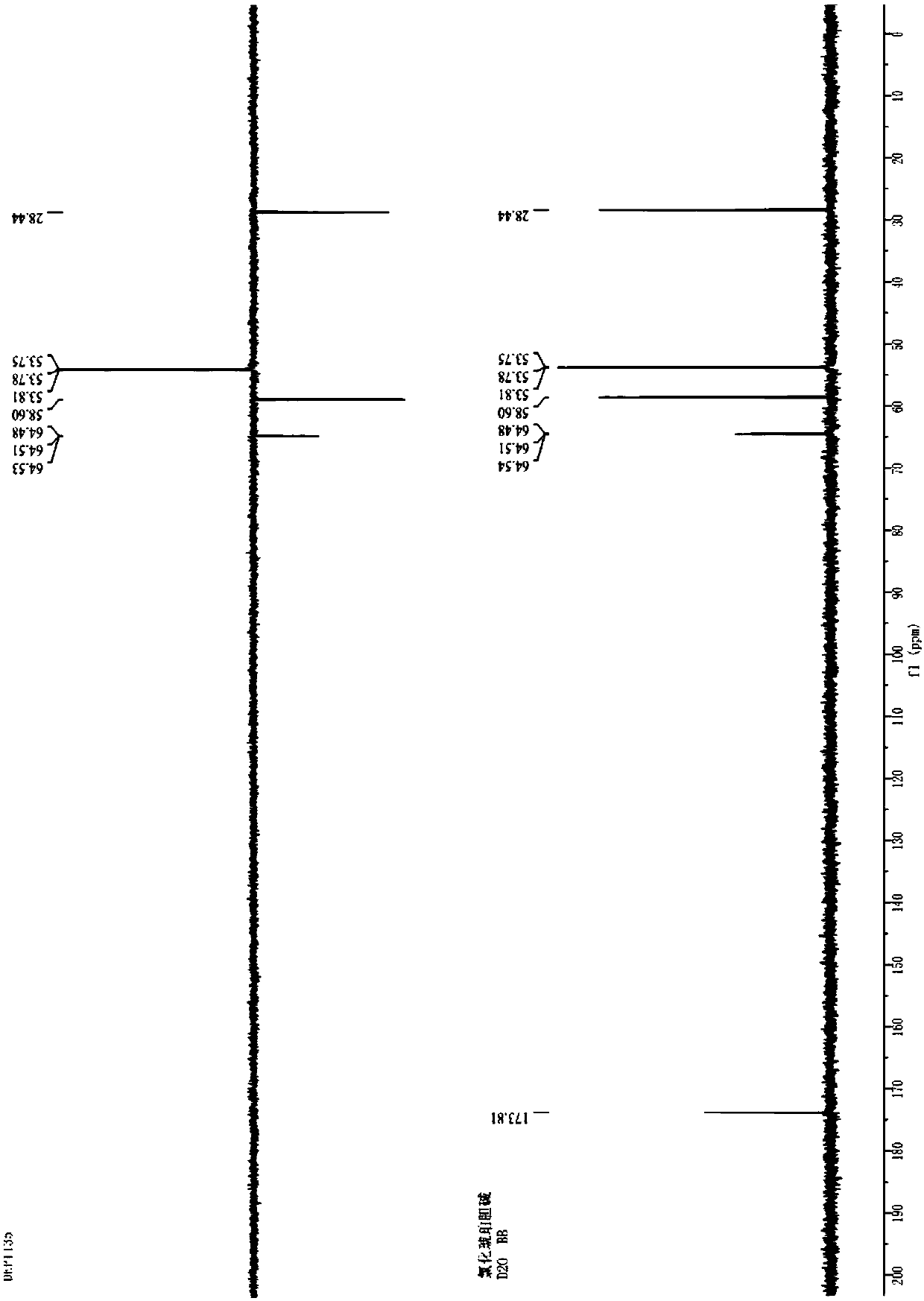
Image
Examples
preparation example Construction
[0035] The preparation method of the present invention may include the following steps:
[0036] (1) Under solvent-free conditions, succinic acid and phosphorus pentachloride are reacted to form a mixture containing succinyl chloride; the mixture containing succinyl chloride is distilled under reduced pressure to collect succinyl chloride;
[0037] (2) Under anhydrous conditions, add dropwise or batchwise the succinyl chloride obtained in step (1) to the mixture of choline chloride and alcohol-free chloroform, and then react to form a mixture containing succinyl chloride The mixture containing succinylcholine chloride is neutralized, dissolved and precipitated to obtain succinylcholine chloride.
[0038] Preferably, the succinyl chloride is added dropwise, and the temperature range of the reaction liquid during dropwise addition is 50-60°C.
[0039] The preparation method of the present invention may also include the following steps:
[0040] (1-1) After reacting succinic acid and phos...
Embodiment 1
[0057] Put the stir bar in the reaction flask (1L) connected with the condenser, drying device, and lye absorption device, and add PCl in batches (3 times) 5 (277.7g×3,833.0g, 4.0mol), succinic acid (78.7g×3,236.2g, 2.0mol). Mix the reactants in the first batch with a glass rod and react at room temperature. After the reactants are basically liquefied and no obvious gas is generated, put in the second batch of PCl 5 React with succinic acid at room temperature. After the reaction solution has no obvious gas generation, put in the third batch of PCl 5 React with succinic acid at room temperature. After the reaction solution has no obvious gas generation, it is heated to 60°C for 1 hour. Collect POCl separately by vacuum distillation 3 And succinyl chloride (water pump decompression, oil bath heating temperature 60-80℃, fraction temperature 32-36℃, collect by-product POCl 3 , 556g, yield 90.7%; oil bath heating temperature 120-125℃, fraction temperature 36-97℃, collect POCl 3 And s...
Embodiment 2
[0062] Pour PCl into the reactor (20L) connected with condenser, stirrer, and lye absorption device in batches (3 times) 5 (5.29kg×3, 15.87kg, 76.21mol), succinic acid (1.50kg×3, 4.50kg, 38.11mol). Mix the reactants in the first batch with a stirrer, and react at room temperature. After the reactants are basically liquefied and no obvious gas is generated, put in the second batch of PCl 5 React with succinic acid at room temperature. After the reactant liquid has no obvious gas generation, put in the third batch of PCl 5 And succinic acid. React at room temperature. After the reaction solution has no obvious gas generation, it is heated to 60°C and stirred for 1 hour. Collect POCl separately by vacuum distillation 3 And succinyl chloride (water pump decompression, oil bath heating temperature 80-100℃, fraction temperature 40-76℃, collect by-product POCl 3 , 11.09kg, yield 94.9%; oil bath heating temperature 100-140℃, distillate temperature 76-95℃, collect POCl 3 And succinyl chl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com