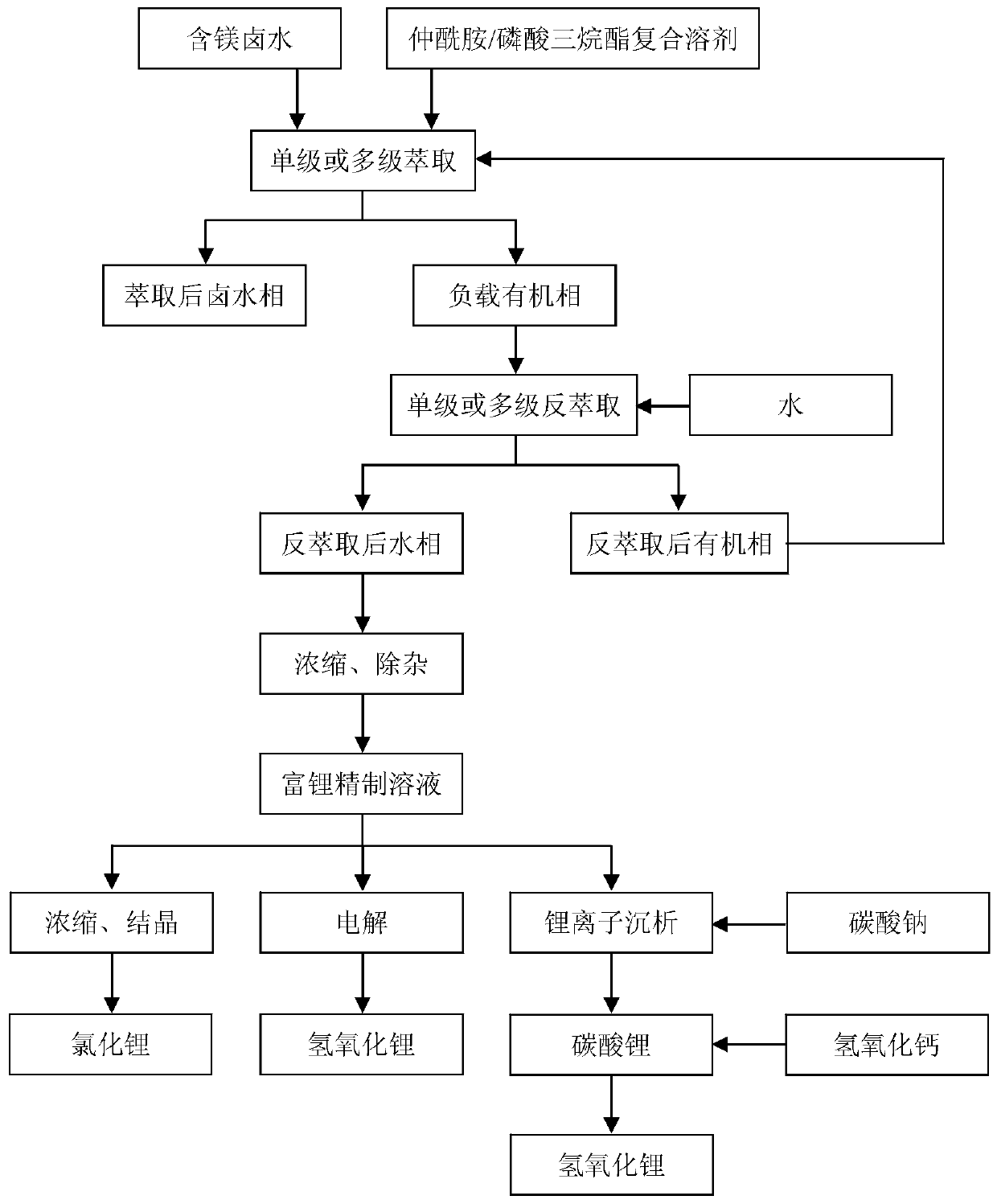
Extraction system and method for separating magnesium from magnesium-containing brine to extract lithium through secondary amide/trialkyl phosphate composite solvent, and application of extraction method
A technology of trialkyl phosphate and composite solvent, which is applied in the direction of lithium halide, lithium carbonate;/acid carbonate, process efficiency improvement, etc., can solve the problem that the extraction system has not been discovered, and the large-scale test of the extraction agent has not been completed Verification and screening and other issues to achieve the effect of reducing acid and alkali consumption, simple structure, and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Li in brine of a salt lake in Qaidam Basin, Qinghai + and Mg 2+ The content is 2.00g / L and 113.43g / L respectively, the mass ratio of magnesium and lithium is 56.67:1, of which Na + , K + , Cl - , and B 2 O 3 The contents are 3.83, 1.60, 325.98, 44.00 and 8.14g / L respectively, and the brine density is 1.34g / cm 3 , the pH value of brine is 4.3. Get 10mL this kind of bittern in 100mL ground conical flask, then add 21mL N-iso-octyl butanamide as extractant and 9mL tributyl phosphate as coextractant, organic phase and salt lake brine volume ratio are 3: 1. Put the magnet in the conical flask, insert the matching air condenser tube into the bottle mouth to prevent the liquid from splashing, put it in the DF-101S type heat-collecting constant temperature heating magnetic stirrer, mix and stir at 20℃, and extract for 20min. Then, the mixed liquid was transferred to a 250mL plastic test tube, and centrifuged at 4500r / min for 15min in an LD5-10 desktop centrifuge. Tran...
Embodiment 2
[0058] Get 21mL N-isoamyl octanamide as extractant and 9mL triisooctyl phosphate as coextractant in 100mL ground conical flask, then add 10mL salt lake brine in Example 1, organic phase and salt lake brine The volume ratio is 3:1. Put the magnet in the conical flask, insert the matching air condenser tube into the bottle mouth to prevent the liquid from splashing, put it in the DF-101S type heat-collecting constant temperature heating magnetic stirrer, mix and stir at 20 ℃, and extract for 30 minutes. Then the mixed liquid was transferred to a 250mL plastic test tube, and centrifuged at 4500r / min for 15min in an LD5-10 desktop centrifuge. Transfer the loaded organic phase to another 100mL ground-mouth conical flask, add deionized water in a volume ratio of 1:6 to the organic phase, and place it in a DF-101S collector-type constant temperature heating magnetic stirrer. Back-extraction was carried out at °C, and the two phases were mixed for 30 min. The mixed liquid was then t...
Embodiment 3
[0064] Get 24mL N-isoamyl octanamide as extractant and 6mL triisodecyl phosphate as coextractant in 100mL ground conical flask, then add 10mL salt lake brine in Example 1, organic phase and salt lake brine The volume ratio is 3:1. Put the magnet in the conical flask, insert the matching air condenser tube into the bottle mouth to prevent the liquid from splashing, put it in the DF-101S type heat-collecting constant temperature heating magnetic stirrer, mix and stir at 20 ℃, and extract for 30 minutes. Then, the mixed liquid was transferred to a 250mL plastic test tube, and centrifuged at 4500r / min for 15min in an LD5-10 desktop centrifuge. Transfer the loaded organic phase to another 100mL ground-mouth conical flask, add deionized water in a volume ratio of 1:4 to the organic phase, and place it in a DF-101S collector type constant temperature heating magnetic stirrer. Back-extraction was carried out at °C, and the two phases were mixed for 30 min. The mixed liquid was then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com