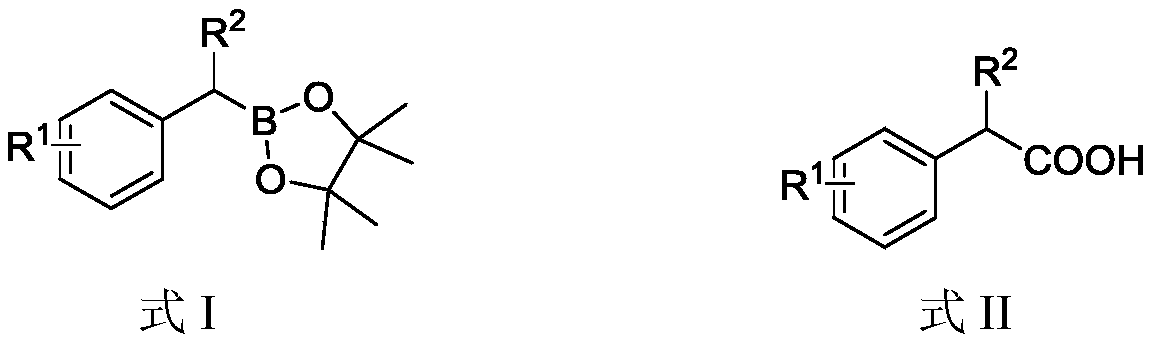Method for converting benzyl borate compounds into phenylacetic acid and derivatives thereof by carbon dioxide
A technology of benzyl borate and carbon dioxide, which is applied in the field of synthesis of phenylacetic acid and its derivatives, can solve the problems of expensive metal reducing agents and air sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
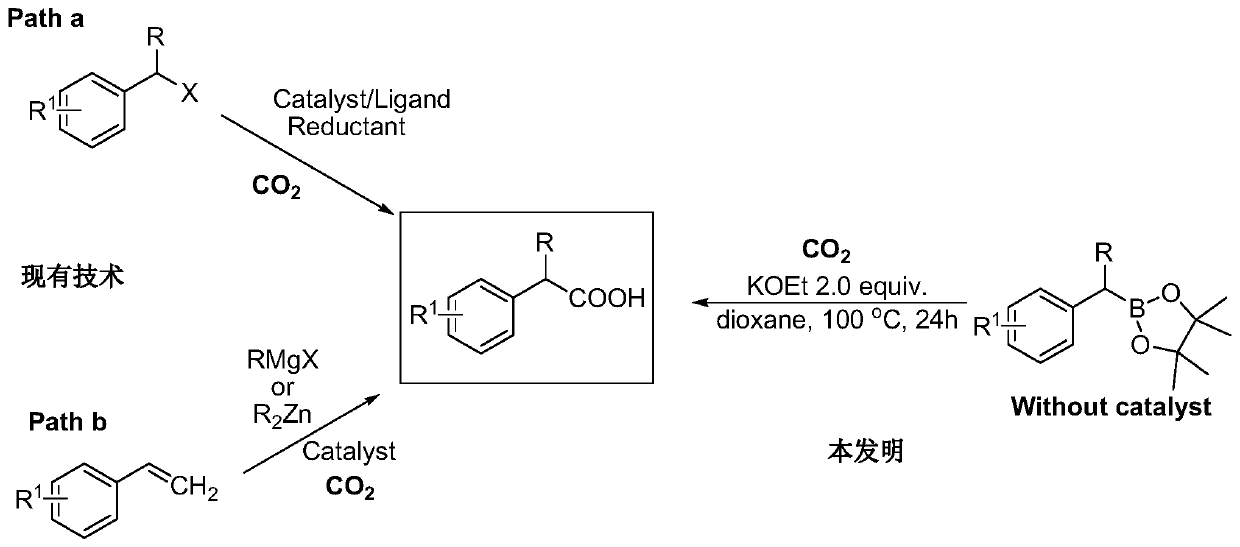
[0027] Add benzylboronic acid pinacol ester (0.3mmol, 1 equivalent, 65.4mg), potassium ethoxide (0.6mmol, 50.5mg), 5mL dioxane to the 100mL reaction flask equipped with a stirring bar successively, in a liquid nitrogen bath The solvent was lyophilized, gas exchanged three times with carbon dioxide, and the reaction bottle was sealed, heated to 100°C and stirred for 24 hours. After the reaction was over, the reaction mixture was removed from the solvent under reduced pressure, the mixture was transferred to a 125mL separatory funnel through ethyl acetate, 5mL dilute hydrochloric acid (1mol / L) was added, 40mL ethyl acetate and 30mL water were added to extract three times, and the combined The organic phase, the solvent was removed under reduced pressure, and purified by column chromatography to obtain the desired product, phenylacetic acid, with a yield of 80%.
Embodiment 2
[0029] Add 2-naphthylmethylboronic acid pinacol ester (0.3mmol, 1 equivalent, 80.5mg), potassium ethoxide (0.6mmol, 50.5mg), 5mL dioxane to a 100mL reaction flask equipped with a stirring bar successively, The solvent was freeze-dried in a liquid nitrogen bath, gas exchanged three times with carbon dioxide, and the reaction bottle was sealed, heated to 100° C. and stirred for 24 hours. After the reaction was over, the reaction mixture was removed from the solvent under reduced pressure, the mixture was transferred to a 125mL separatory funnel through ethyl acetate, 5mL dilute hydrochloric acid (1mol / L) was added, 40mL ethyl acetate and 30mL water were added to extract three times, and the combined The organic phase, the solvent was removed under reduced pressure, and purified by column chromatography to obtain the desired product 2-naphthylacetic acid with a yield of 60%.
Embodiment 3
[0031] Add 4-phenylbenzylboronic acid pinacol ester (0.3mmol, 1 equivalent, 88.3mg), potassium ethoxide (0.6mmol, 50.5mg), 5mL dioxane to a 100mL reaction flask equipped with a stirring bar successively , freeze-dried the solvent in a liquid nitrogen bath, gas exchanged three times with carbon dioxide, sealed the reaction bottle, heated to 100 ° C and stirred for 24 hours. After the reaction was over, the reaction mixture was removed from the solvent under reduced pressure, the mixture was transferred to a 125mL separatory funnel through ethyl acetate, 5mL dilute hydrochloric acid (1mol / L) was added, 40mL ethyl acetate and 30mL water were added to extract three times, and the combined The organic phase, the solvent was removed under reduced pressure, and purified by column chromatography to obtain the desired product 4-phenylphenylacetic acid with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com