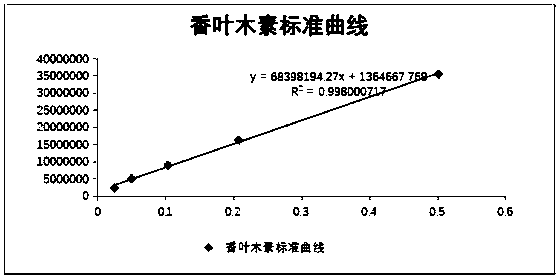
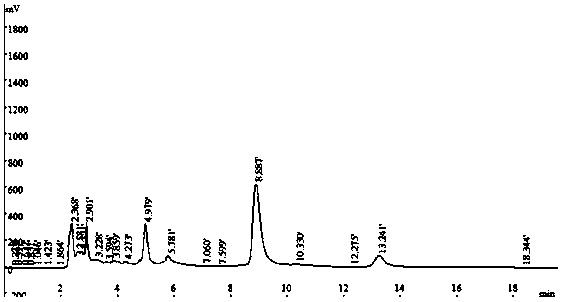
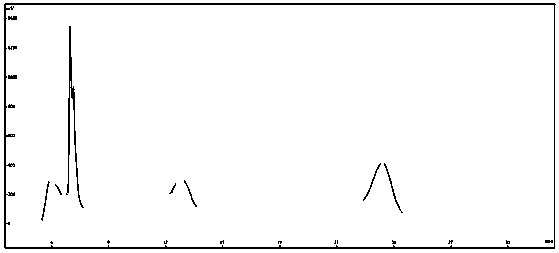
Method for preparation of diosmetin from peanut shells
A technology of geranialignin and peanut shells, applied in the direction of organic chemistry, etc., can solve the problems of extracting geranialignin, reduce the cost of compound raw materials, etc., and achieve the effect of increasing the concentration and expanding the source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Weigh 5g of peanut shell powder, extract at a material-to-liquid ratio of 1:15, add 75mL of 70% ethanol to it, extract at 80°C, heat in microwave for 10min, leave the filter residue after suction filtration and extract again under the same conditions, after the second extraction Combine the filtrates.
[0027] (2) Add 4 mL of 0.48 mol / L ferric chloride salt solution into the peanut shell extract obtained in step (1), carry out stirring reaction at room temperature, and then filter to obtain a clear filtrate. The reaction time is more than 1 hr.
[0028] (3) Preparative chromatographic conditions: the chromatographic column is a C18 chromatographic column (20 × 250mm, 5um), the mobile phase is methanol: water = 65:35, the flow rate is 12ml / min, the detection wavelength: 254nm, the column temperature: room temperature, and the injection volume : 2ml. Use preparative high performance liquid chromatography to separate the filtrate obtained in step (2), collect the chr...
Embodiment 2
[0030] (1) Weigh 5 g of peanut shell powder, extract with a material-to-liquid ratio of 1:10, add 50 mL of 70% ethanol to it, extract at a temperature of 80 ° C, and heat with microwave for 20 min. After suction filtration, the filter residue is extracted again under the same conditions. Combine the filtrates.
[0031] (2) Add 4 mL of 0.48 mol / L ferric chloride salt solution into the peanut shell extract obtained in step (1), carry out stirring reaction at room temperature, and then filter to obtain a clear filtrate. The reaction time is more than 1 hr.
[0032] (3) Preparative chromatographic conditions: the chromatographic column is a C18 chromatographic column (20 × 250mm, 5um), the mobile phase is methanol: water = 65:35, the flow rate is 12ml / min, the detection wavelength: 254nm, the column temperature: room temperature, and the injection volume : 2ml. Use preparative high performance liquid chromatography to separate the filtrate obtained in step (2), collect the chrom...
Embodiment 3
[0034] (1) Weigh 5 g of peanut shell powder, extract with a material-to-liquid ratio of 1:20, add 100 mL of 70% ethanol to it, extract at 80 ° C, and heat with microwave for 2 min. After suction filtration, the filter residue is extracted again under the same conditions. Combine the filtrates.
[0035] (2) Add 4 mL of 0.48 mol / L ferric chloride salt solution into the peanut shell extract obtained in step (1), carry out stirring reaction at room temperature, and then filter to obtain a clear filtrate. The reaction time is more than 1 hr.
[0036] (3) Preparative chromatographic conditions: the chromatographic column is a C18 chromatographic column (20 × 250mm, 5um), the mobile phase is methanol: water = 65:35, the flow rate is 12ml / min, the detection wavelength: 254nm, the column temperature: room temperature, and the injection volume : 2ml. Use preparative high performance liquid chromatography to separate the filtrate obtained in step (2), collect the chromatographic peaks ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com