Preparation method of electronic-grade boron trichloride
A boron trichloride, electronic grade technology, applied in the direction of boron halide compounds, boron halides, etc., can solve the problems of unfriendly environment, no explanation of the purity of boron trichloride raw materials, boron trichloride purity, etc., to reduce the difficulty of refining , Reduce the generation of phosgene and water, reduce the effect of energy consumption and cooling consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
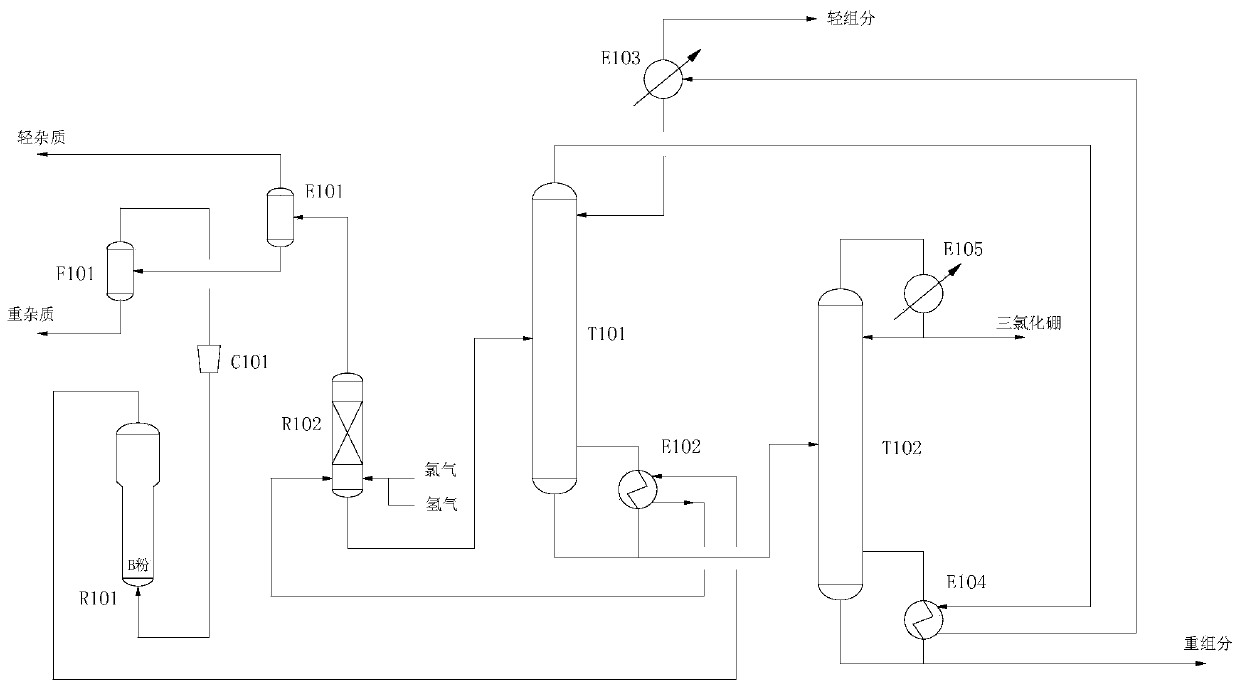
[0036] Boron powder and hydrogen chloride after heat exchange react in the ebullating bed reactor R101. The temperature of the ebullating bed reactor R101 is 300°C and the pressure is 1MPa. After heat exchange, it enters the photocatalytic reactor R102. Chlorine gas is charged from the bottom of the photocatalytic reactor. The molar ratio of boron powder and chlorine gas is 1:1.5. At the same time, an appropriate amount of hydrogen gas is charged to ensure that all the chlorine gas reacts to form hydrogen chloride. The temperature of the photocatalytic reactor R102 is 30°C and the pressure is 0.4MPa. The gas phase from the top of the photocatalytic reactor R102 enters the HCl cold trap E101, and the crude boron trichloride from the bottom of the photocatalytic reactor enters the light removal tower T101. Uncondensed light impurities are extracted from the top of the HCl cold trap E101, hydrogen chloride is extracted from the lower part and enters the vaporizer F101, gas phase ...
Embodiment 2
[0041]Boron powder and hydrogen chloride after heat exchange react in the ebullating bed reactor R101. The temperature of the ebullating bed reactor R101 is 500°C and the pressure is 3MPa. After heat exchange, it enters the photocatalytic reactor R102. Chlorine gas is charged from the bottom of the photocatalytic reactor. The molar ratio of boron powder and chlorine gas is 1:2. At the same time, an appropriate amount of hydrogen gas is charged to ensure that all the chlorine gas reacts to generate hydrogen chloride. The temperature of the photocatalytic reactor R102 is 50°C and the pressure is 0.8MPa. The gas phase from the top of the photocatalytic reactor R102 enters the HCl cold trap E101, and the crude boron trichloride from the bottom of the photocatalytic reactor enters the light removal tower T101. Uncondensed light impurities are extracted from the top of the HCl cold trap E101, hydrogen chloride is extracted from the lower part and enters the vaporizer F101, gas phase...
Embodiment 3
[0046] Boron powder and hydrogen chloride after heat exchange react in the ebullating bed reactor R101. The temperature of the ebullating bed reactor R101 is 500°C and the pressure is 5MPa. After heat exchange, it enters the photocatalytic reactor R102. Chlorine gas is charged from the bottom of the photocatalytic reactor. The molar ratio of boron powder and chlorine gas is 1:3. At the same time, an appropriate amount of hydrogen gas is charged to ensure that all the chlorine gas reacts to form hydrogen chloride. The temperature of the photocatalytic reactor R102 is 60°C and the pressure is 1MPa. The gas phase from the top of the photocatalytic reactor R102 enters the HCl cold trap E101, and the crude boron trichloride from the bottom of the photocatalytic reactor enters the light removal tower T101. Uncondensed light impurities are extracted from the top of the HCl cold trap E101, hydrogen chloride is extracted from the lower part and enters the vaporizer F101, gas phase hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com